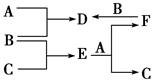
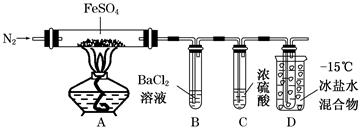
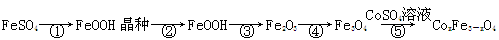��Ŀ����
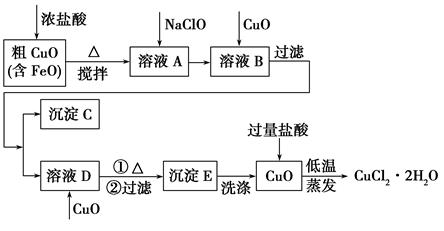
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ��������ˮ���Ҵ���ϡ���ᣬ�۵�422
�棬�е�1366 �棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ����������£�
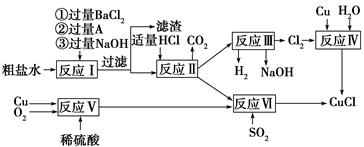
(1)A�Ļ�ѧʽΪ________��
(2)д����Ӧ���Ļ�ѧ����ʽ______________________________________
(3)д����Ӧ�������ӷ���ʽ______________________________________
(4)�������п���ѭ�����õ�������(�û�ѧʽ��ʾ)___________________________
(5)��Ӧ�����˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 �� ��2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70 ����ո����Ŀ����_________________________
�棬�е�1366 �棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ����������£�

(1)A�Ļ�ѧʽΪ________��
(2)д����Ӧ���Ļ�ѧ����ʽ______________________________________
(3)д����Ӧ�������ӷ���ʽ______________________________________
(4)�������п���ѭ�����õ�������(�û�ѧʽ��ʾ)___________________________
(5)��Ӧ�����˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 �� ��2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70 ����ո����Ŀ����_________________________
(1)Na2CO3
(2)2Cu��O2��2H2SO4=2CuSO4��2H2O
(3)2Cu2����2Cl����SO2��2H2O=2CuCl����4H����SO42��
(4)NaOH��H2SO4
(5)�ӿ��Ҵ���ˮ����������ֹCuCl����������
(2)2Cu��O2��2H2SO4=2CuSO4��2H2O
(3)2Cu2����2Cl����SO2��2H2O=2CuCl����4H����SO42��
(4)NaOH��H2SO4
(5)�ӿ��Ҵ���ˮ����������ֹCuCl����������
�Ʊ����̻��漰�������ᴿ��ʵ�飬Ҫע���������ۺϿ��ǡ�Ϊ��ʹ���ʾ���������һ�����ij����Լ���Ҫ�����������ij����Լ��ں�ߵIJ����б���ȥ��

��ϰ��ϵ�д�
�����Ŀ

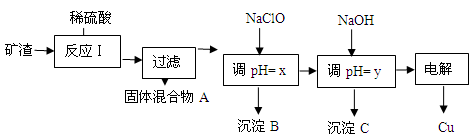
 HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��