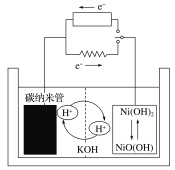
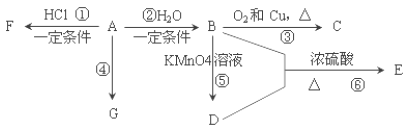
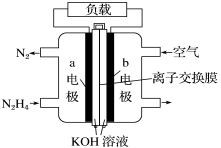
��Ŀ����
����Ŀ��(NH4)2Cr2O7�������л��ϳɴ�����ýȾ������ӰҺ�ȡ�ij��ѧ��ȤС���(NH4)2Cr2O7�IJ������ʼ���ɽ���̽������֪��Cr2O72-(��ɫ)+H2O=2Cr2O42-(��ɫ)+2H+����ش��������⣺
(1)���Թ��м�������(NH4)2Cr2O7���壬�μ�����ŨKOH��Һ�����ȣ��۲쵽����Ҫ������___��
(2)Ϊ̽��(NH4)2Cr2O7(Ħ������Ϊ252g/mol)�ķֽ�������ͼ���Ӻ�װ�ã���A�м���5.040g��Ʒ����ʵ�顣

������B��������___��
��C��������___��
�۷�Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ����___��
�ܼ���A�����أ��۲쵽D����Һ����ɫ��ͬʱ���A��B�������ı仯�ֱ�Ϊ200g��1.44g��д���ظ���識��ȷֽⷴӦ�Ļ�ѧ����ʽ��___��
(3)ʵ���ҳ��ü�ȩ���ⶨ��(NH4)2Cr2O7����Ʒ�е��������������䷴Ӧԭ��Ϊ 2Ba2++Cr2O72-+H2O=2BaCrO4��+2H+��4NH4++6HCHO=3H++6H2O+(CH2)6N4H�ζ�ʱ��1mo1(CH2)N4H+��1mo1H+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣ
ʵ�鲽�裺��ȡ��Ʒ2.800g�����250mL��Һ����ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У����Ȼ�����ҺʹCr2O72-��ȫ��������10mL2.000 mo1L-1�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����0.200mo1L-1NaOH ����Һ�ζ����յ㡣�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ20.00mL��
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ�����ζ�ʱ��ȥ��NaOH����Һ�����___(����ƫ������ƫС��������Ӱ����)��
�ڵζ�����ø���Ʒ�е�����������Ϊ___��
���𰸡������ܽ⣬������ɫ���д̼�����ζ�����壬��Һ�ɳ�ɫ��Ϊ��ɫ U����� ��ֹD��ˮ��������B�У����Ų�������ˮ���������� ��A�зֽ����������ȫ������B�У���ֹ����ʵ����� (NH4)2Cr2O7![]() Cr2O3+N2��+4H2O ƫ�� 10.00%
Cr2O3+N2��+4H2O ƫ�� 10.00%
![]() ���Թ��м�������
���Թ��м�������![]() ���壬�μ�����ŨKOH��Һ�����ȣ���֪��
���壬�μ�����ŨKOH��Һ�����ȣ���֪��![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() ����Һ��ƽ��������У���Һ�Ի�ɫ���۲쵽����Ҫ�����ǣ������ܽ⣻������ɫ���д̼�����ζ�����壻��Һ�ɳ�ɫ��Ϊ��ɫ��
����Һ��ƽ��������У���Һ�Ի�ɫ���۲쵽����Ҫ�����ǣ������ܽ⣻������ɫ���д̼�����ζ�����壻��Һ�ɳ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ�������ܽ⣬������ɫ���д̼�����ζ�����壬��Һ�ɳ�ɫ��Ϊ��ɫ��
![]() ����B������ΪU����ܣ�
����B������ΪU����ܣ�
�ʴ�Ϊ��U����ܣ�
![]() �������Ƿ�ֹD��ˮ��������B�У����Ų�������ˮ������������
�������Ƿ�ֹD��ˮ��������B�У����Ų�������ˮ������������
�ʴ�Ϊ����ֹD��ˮ��������B�У����Ų�������ˮ������������
![]() ��Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ���ǣ���A�зֽ����������ȫ������B�У���ֹ����ʵ����
��Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ���ǣ���A�зֽ����������ȫ������B�У���ֹ����ʵ����
�ʴ�Ϊ����A�зֽ����������ȫ������B�У���ֹ����ʵ����
![]() Ħ������Ϊ
Ħ������Ϊ![]() �ķֽ�����A�м���
�ķֽ�����A�м���![]() ��Ʒ�����ʵ���Ϊ
��Ʒ�����ʵ���Ϊ![]() ������A�����أ��۲쵽D����Һ����ɫ��˵���ް��������ɣ����ɵ��ǵ�����ʱ���A��B�������ı仯�ֱ�Ϊ200g��
������A�����أ��۲쵽D����Һ����ɫ��˵���ް��������ɣ����ɵ��ǵ�����ʱ���A��B�������ı仯�ֱ�Ϊ200g��![]() ��A�в�����Ϊ
��A�в�����Ϊ![]() �������ʵ���ΪB�������ı仯Ϊ
�������ʵ���ΪB�������ı仯Ϊ![]() ��Ϊ���յ�ˮ�������ʵ���Ϊ
��Ϊ���յ�ˮ�������ʵ���Ϊ![]() ���������غ㶨�ɿ�֪��ѧ����ʽΪ
���������غ㶨�ɿ�֪��ѧ����ʽΪ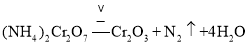 ��
��
�ʴ�Ϊ��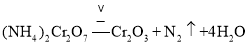 ��
��
![]() ��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ�ⶨ���ƫ�ߣ�
��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ�ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ��
![]() ��Ӧԭ��Ϊ
��Ӧԭ��Ϊ![]() ��
��![]() �ζ�ʱ��
�ζ�ʱ��![]() ��
��![]() �൱
�൱![]() ��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��
��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��![]() ����Ʒ���ʵ���Ϊx�������
����Ʒ���ʵ���Ϊx�������![]() ����������2xmol��笠��ͼ�ȩ��Ӧ�����������൱��
����������2xmol��笠��ͼ�ȩ��Ӧ�����������൱��![]() ��Ӧ����
��Ӧ����![]() ����
����![]() ����Һ�ζ����յ㣬�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ
����Һ�ζ����յ㣬�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ![]() ��
��
��![]() �����
�����![]() ������Ʒ�е�����������Ϊ
������Ʒ�е�����������Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��������
![]() ���Թ��м�������
���Թ��м�������![]() ���壬�μ�����ŨKOH��Һ��һ���棬
���壬�μ�����ŨKOH��Һ��һ���棬![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() ����Һ��ƽ��������У���Һ�Ի�ɫ����һ���棬NH4++OH-=NH3��+H2O���ɴ˵ó��۲쵽����Ҫ����
����Һ��ƽ��������У���Һ�Ի�ɫ����һ���棬NH4++OH-=NH3��+H2O���ɴ˵ó��۲쵽����Ҫ����
![]() ����B������ΪU����ܣ�
����B������ΪU����ܣ�
![]() �������Ƿ�ֹD��ˮ��������B�У�
�������Ƿ�ֹD��ˮ��������B�У�
![]() ��Ӧ������װ��������һ�����ķ�Ӧ�������壬�轫���ų���
��Ӧ������װ��������һ�����ķ�Ӧ�������壬�轫���ų���
![]() Ħ������Ϊ
Ħ������Ϊ![]() �ķֽ�����A�м���
�ķֽ�����A�м���![]() ��Ʒ�����ʵ���Ϊ
��Ʒ�����ʵ���Ϊ![]() ������A�����أ��۲쵽D����Һ����ɫ��˵���ް������ɣ����ɵ��ǵ��������A��B�������ı仯�ֱ�Ϊ200g��
������A�����أ��۲쵽D����Һ����ɫ��˵���ް������ɣ����ɵ��ǵ��������A��B�������ı仯�ֱ�Ϊ200g��![]() �������A�в�����
�������A�в�����![]() �����ʵ�����B�������ı仯Ϊ
�����ʵ�����B�������ı仯Ϊ![]() ����������յ�ˮ���������ʵ������������غ㶨�ɿɵó���ѧ����ʽ��
����������յ�ˮ���������ʵ������������غ㶨�ɿɵó���ѧ����ʽ��
![]() ��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ���ɴ˷��������
��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ���ɴ˷��������
![]() ��Ӧԭ��Ϊ
��Ӧԭ��Ϊ![]() ��
��![]() �ζ�ʱ��
�ζ�ʱ��![]() ��
��![]() �൱
�൱![]() ��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��
��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��![]() ����Ʒ���ʵ���Ϊx������H++OH-=H2O�Ķ�����ϵ������������ϵʽ���Ӷ��������Ʒ�е�������������
����Ʒ���ʵ���Ϊx������H++OH-=H2O�Ķ�����ϵ������������ϵʽ���Ӷ��������Ʒ�е�������������
![]() ���Թ��м�������
���Թ��м�������![]() ���壬�μ�����ŨKOH��Һ�����ȣ���֪��
���壬�μ�����ŨKOH��Һ�����ȣ���֪��![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() ����Һ��ƽ��������У���Һ�Ի�ɫ���۲쵽����Ҫ�����ǣ������ܽ⣻������ɫ���д̼�����ζ�����壻��Һ�ɳ�ɫ��Ϊ��ɫ����Ϊ�������ܽ⣬������ɫ���д̼�����ζ�����壬��Һ�ɳ�ɫ��Ϊ��ɫ��
����Һ��ƽ��������У���Һ�Ի�ɫ���۲쵽����Ҫ�����ǣ������ܽ⣻������ɫ���д̼�����ζ�����壻��Һ�ɳ�ɫ��Ϊ��ɫ����Ϊ�������ܽ⣬������ɫ���д̼�����ζ�����壬��Һ�ɳ�ɫ��Ϊ��ɫ��
![]() ����B������ΪU����ܣ���Ϊ��U����ܣ�
����B������ΪU����ܣ���Ϊ��U����ܣ�
![]() �������Ƿ�ֹD��ˮ��������B�У����Ų�������ˮ��������������Ϊ����ֹD��ˮ��������B�У����Ų�������ˮ������������
�������Ƿ�ֹD��ˮ��������B�У����Ų�������ˮ��������������Ϊ����ֹD��ˮ��������B�У����Ų�������ˮ������������
![]() ��Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ���ǣ���A�зֽ����������ȫ������B�У���ֹ����ʵ������Ϊ����A�зֽ����������ȫ������B�У���ֹ����ʵ����
��Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ���ǣ���A�зֽ����������ȫ������B�У���ֹ����ʵ������Ϊ����A�зֽ����������ȫ������B�У���ֹ����ʵ����
![]() Ħ������Ϊ
Ħ������Ϊ![]() �ķֽ�����A�м���
�ķֽ�����A�м���![]() ��Ʒ�����ʵ���Ϊ
��Ʒ�����ʵ���Ϊ![]() ������A�����أ��۲쵽D����Һ����ɫ��˵���ް������ɣ����ɵ��ǵ��������A��B�������ı仯�ֱ�Ϊ200g��
������A�����أ��۲쵽D����Һ����ɫ��˵���ް������ɣ����ɵ��ǵ��������A��B�������ı仯�ֱ�Ϊ200g��![]() ��A�в�����Ϊ
��A�в�����Ϊ![]() �������ʵ���Ϊ0.01mol��B�������ı仯Ϊ
�������ʵ���Ϊ0.01mol��B�������ı仯Ϊ![]() ��Ϊ���յ�ˮ�������ʵ���Ϊ
��Ϊ���յ�ˮ�������ʵ���Ϊ![]() ���������غ㶨�ɿ�֪��ѧ����ʽΪ
���������غ㶨�ɿ�֪��ѧ����ʽΪ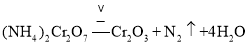 ����Ϊ��
������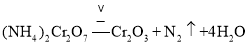 ��
��
![]() ��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ�ⶨ���ƫ��Ϊ��ƫ��
��ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ����൱��ϡ�ͣ���ζ�ʱ��ȥ��NaOH����Һ�����ƫ�ⶨ���ƫ��Ϊ��ƫ��
![]() ��Ӧԭ��Ϊ
��Ӧԭ��Ϊ![]() ��
��![]() �ζ�ʱ��
�ζ�ʱ��![]() ��
��![]() �൱
�൱![]() ��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��
��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬ��![]() ����Ʒ���ʵ���Ϊx�������
����Ʒ���ʵ���Ϊx�������![]() ����������2xmol��笠��ͼ�ȩ��Ӧ�����������൱��
����������2xmol��笠��ͼ�ȩ��Ӧ�����������൱��![]() ��Ӧ����
��Ӧ����![]() ����
����![]() ����Һ�ζ����յ㣬�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ
����Һ�ζ����յ㣬�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ![]() ����
����![]() �����
�����![]() ������Ʒ�е�����������Ϊ
������Ʒ�е�����������Ϊ![]() ����Ϊ��
������![]() ��
��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�����Ŀ���¶�ΪT1ʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ: 2NO2(g)![]() 2NO(g)+O2(g) (����Ӧ����)��ʵ����:v��=v(NO2)����=k��c2(NO2)��v��=v(NO)����= 2v(O2)����=k��c2(NO)��c(O2)��k����k��Ϊ���ʳ��������¶�Ӱ�졣
2NO(g)+O2(g) (����Ӧ����)��ʵ����:v��=v(NO2)����=k��c2(NO2)��v��=v(NO)����= 2v(O2)����=k��c2(NO)��c(O2)��k����k��Ϊ���ʳ��������¶�Ӱ�졣
����˵����ȷ����
���� ��� | ���ʵ���ʼŨ�ȣ�mol��L��1�� | ���ʵ�ƽ��Ũ�ȣ�mol��L��1�� | ||
c(NO2) | c(NO) | c(O2) | c(O2) | |
�� | 0.6 | 0 | 0 | 0.2 |
�� | 0.3 | 0.5 | 0.2 | |
�� | 0 | 0.5 | 0.35 | |
A. ��kΪ�÷�Ӧ�Ļ�ѧƽ�ⳣ��������k=k��:k��
B. ��ƽ��ʱ�����������������е���ѹǿ֮��Ϊ20��17
C. ����������ʼƽ�������ƶ�����ƽ��ʱ����������NO2��ת���ʱ��������е�С
D. ���ı��¶�ΪT2,��T2>T1,��k��:k��<0.8