��Ŀ����
����Ŀ��CO2��һ���������壬����������滷���������Ӱ�죬ά�ִ�����CO2��ƽ�����̬��������������Ҫ���塣
��1��CO2����ϳɵ�̼ϩ����������Ч����CO2���Ժϳ�C2H4Ϊ������ת����Ϊ�������У�
��һ����CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H1=+41.3kJ/mol
CO(g)+H2O(g) ��H1=+41.3kJ/mol
�ڶ�����2CO(g)+4H2(g)![]() C2H4(g)+2H2O(g) ��H2=-210.5kJ/mol
C2H4(g)+2H2O(g) ��H2=-210.5kJ/mol
CO2����ϳ���ϩ���Ȼ�ѧ����ʽΪ__��
��2������CO2��H2�ϳɼ״�����һ����Ч����CO2��;������Ӧ���£�CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H3
CH3OH(g)+H2O(g) ��H3
�����ݻ�Ϊ2L�ĺ����ܱ������У�ͨ��2molCO2��3molH2����������Ӧ������˵���ܹ������ÿ��淴Ӧ�ﵽƽ��״̬����__������ĸ��
a.����1.5molH2ʱ����0.5molCH3OH����
b.ת��3mol����ʱ������11.2L����״���£�CO2
c.��ϵ��������ܶȲ���
d.ˮ����������������ֲ���
e.��λʱ��������H2������H2O�����ʵ���֮��Ϊ3��1
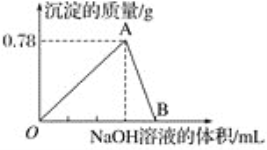
���о��¶ȶԸ÷�Ӧ�״����ʵ�Ӱ�졣��210�桫290�棬����ԭ������CO2��H2��Ͷ�ϱȲ��䣬��һ�����ٷ���������Ӧ���õ��״�ƽ��������¶ȵĹ�ϵ��ͼ��ʾ����H3__0�����������=�����������ж�������__��

����һ�̶��ݻ����ܱ������з���������Ӧ����Ҫ���ƽ��ʱCH3OH���ʣ�����Բ�ȡ�Ĵ�ʩ��__������ĸ����
a.���� b.������� c.����CO2��Ũ�� d.����H2��ѹ e.����������� f.������״�
��3����һ���¶Ⱥʹ��������£�Ҳ�ɽ�CO2ת��Ϊȼ��CH4����Ӧ����ʽΪCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)����30��ʱ��һ������CO2��H2����������ݻ�Ϊ1L�ĺ����ܱ������з���������Ӧ��5min��ﵽ��⣬��ʱ�����ʵ�Ũ�����±���
CH4(g)+2H2O(g)����30��ʱ��һ������CO2��H2����������ݻ�Ϊ1L�ĺ����ܱ������з���������Ӧ��5min��ﵽ��⣬��ʱ�����ʵ�Ũ�����±���
���� | CO2(g) | H2(g) | CH4(g) | H2O(g) |
Ũ��/mol��L-1 | 0.2 | 0. | a | 1.6 |
��a=__���÷�Ӧƽ�ⳣ��K=__��
��4����TiO2/Cu2Al2O4Ϊ����������CH4���Խ�CO2ֱ��ת�������ᡣ�ڲ�ͬ�¶��´����Ĵ�Ч����������������ʵĹ�ϵ��ͼ��ʾ�����������������Ҫȡ�����¶�Ӱ��ķ�Χ��__��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����__��

���𰸡�2CO2(g)+6H2(g)=C2H4(g)+4H2O(g) ��H=-127.9 kJ/mol de �� �¶����ߣ��״���ƽ����ʽ���˵������Ӧ���� cdf 0.8 25 300��400�� �¶ȳ���250�棬�����Ĵ�Ч�����Խ���
��������
(1)���ݸ�˹���ɷ�Ӧ1��2+��Ӧ2���Եõ�CO2����ϳ���ϩ�Ļ�ѧ����ʽ����H=��H1��2+��H2=+41.3kJ/mol��2-210.5kJ/mol=-127.9 kJ/mol�����Ȼ�ѧ����ʽΪ2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H=-127.9 kJ/mol��
C2H4(g)+4H2O(g) ��H=-127.9 kJ/mol��
(2)��a.����1.5molH2ʱ����0.5molCH3OH���ɣ����߶�Ϊ����Ӧ���ʣ����ܱ����ﵽƽ��״̬��
b.ת��3mol����ʱ������11.2L����״���£�CO2������˵�����淴Ӧ������ȣ����ܱ�����Ӧ�ﵽƽ��״̬��
c.������������䣬��ϵ��������������䣬������һֱ�ܶȲ��䣬���ܱ�����Ӧ�ﵽƽ��״̬��
d.ˮ����������������ֲ��䣬˵�������Ũ�Ȳ��ٸı䣬�������Ӧ�ﵽƽ��״̬��
e.��λʱ��������H2Ϊ�淴Ӧ��������H2OΪ����Ӧ�����������ɵ����ʵ���֮��Ϊ3��1�������淴Ӧ������ȣ�������Ӧ�ﵽƽ��״̬��
��ѡde��
����ͼ���֪�����¶����״���ƽ����ʽ��ͣ�������ӦΪ���ȷ�Ӧ����H3��0��
��a.��Ϊ����ӦΪ���ȷ�Ӧ������ƽ�������ƶ���ƽ��ʱCH3OH���ʽ��ͣ�
b.������Ӱ�컯ѧƽ�⣬���������ƽ��ʱCH3OH���ʲ��䣻
c.����CO2��Ũ�ȣ�ƽ�������ƶ���ƽ��ʱCH3OH��������
d.����H2��ѹ�����ӷ�Ӧ��Ũ�ȣ�ƽ�������ƶ���ƽ��ʱCH3OH��������
e.����������壬����������䣬��Ӧ�����������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���ƽ��ʱCH3OH���ʲ��䣻
f.������״�����С�������Ũ�ȣ�ƽ�������ƶ���ƽ��ʱCH3OH��������
��ѡcdf��
(3)CO2(g)+4H2(g)![]() CH4(g)+2H2O(g)��
CH4(g)+2H2O(g)��![]() �����ݱ������ݿ�֪����Ӧ����H2O(g)��Ũ��Ϊ1.6 mol��L-1��������c(CH4)=
�����ݱ������ݿ�֪����Ӧ����H2O(g)��Ũ��Ϊ1.6 mol��L-1��������c(CH4)=![]() c(H2O)=0.8 mol��L-1����a=0.8���÷�Ӧƽ�ⳣ��
c(H2O)=0.8 mol��L-1����a=0.8���÷�Ӧƽ�ⳣ��![]() =
=![]() ��
��
��4����ͼ���֪���¶�300��400��ʱ��Ч����ǿ����������������ʽ��ͣ���Ҫ��Ϊ�¶�Ӱ����������������ʣ���ͼ���֪���¶ȳ���250�棬�����Ĵ�Ч�����Խ��ͣ����¶����߶�������������ʽ��͡�

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�