��Ŀ����
����Ŀ��ӡˢͭ�Ƶ�·���ʴ��Һѡȡ�ͻ���������һֱ���о����ȵ㡣
��1��FeCl3��Һһֱ��Ϊ��ͳ��ʴ��Һ��
��ʴ�̹����������ӷ���ʽΪ______________________________��
��ʴ�̽�������ͨ�������ȷ����ͭ��Ƚʵ��FcCl3��Һ������
i.��1�������Լ��Ͳ����ֱ�Ϊ____________________________��
ii.��2��ת���ɼ����������_________________________(��һ�ּ���)��
��2��H2O2Ҳ��������ͭ�Ƶ�·��ʴ��Һ��ʹ��ʱ���������ˮ�������Ƴ����Ի����ʴ��Һ��Ӧ������ʴ��Һ(HCl��H2O2)��������ʴ�̷�Һ�����������£�

��ʴ��ͭ����Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
�ڻ�����Cu2O�����У�������Լ�A��______(����ĸ)��
a.Fe�� b.������ C.NaCl���� d.����KMnO4��Һ
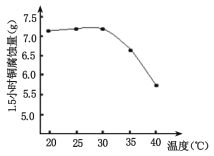
�ۻ���Cu2(OH)2CO3�Ĺ���������Ʒ�Ӧ���¶ȣ����¶��{��80��ʱ����Ʒ��ɫ��������ԭ�������______________________��
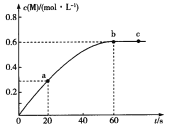
����ͼ���о�����ʴ��Һ���¶ȶ�ﵸ�ʴ����ʵ�����������¶ȣ���ʴ���仯��ԭ��______________________��
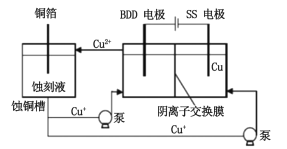
��3���볣�淽����ͬ�����о�����HCl��CuCl2��ʴ��Һ��ʴͭ�����������������Cu+��Һ��������ͼ��ʾ�������ɴﵽʴ��Һ���������ս���ͭ��Ŀ�ġ��˷����ò����������ʯBDD�缫����ֱ�Ӵ�ˮ���γ�һ�־���ǿ�����Ե�������ɻ�(HO��)����д��BDD�缫�ϵĵ缫��Ӧ__________________����һ����Һ�з�Ӧ��ʵ��ʴ��Һ��������д����ʴҺ���������ӷ���ʽ_________________��
���𰸡� 2Fe3++Cu=2Fe2++Cu2+ ����������ۣ����� Cl2(�����𰸺���) Cu+2H++H2O2=Cu2++2H2O b �¶ȸ���80��ʱ,Cu2(OH)2CO3�ֽ����ɺ�ɫCuO,���²�Ʒ��ɫ���� 20-30��ʱ�������¶�����ʴ��������������Ҫ����Ϊ�¶�����ʹ��Ӧ���ʼӿ���30�������������¶�����ʴ����������Ҫʱ��ΪH2O2�ֽ⡢NH3�ӷ� H2O-e-=HO��+H H++Cu++��OH=Cu2++H2O
����������1����FeCl3��Һ��Ϊʴ��Һ��������Fe3+��ǿ�����ԡ�ʴ�̹����������ӷ���ʽΪ2Fe3++Cu=2Fe2++Cu2+��
��ʴ�̽��������������ʹCu2++ Fe= Fe2++Cu�����˺����Һ�ټ�����ˮʹ
Fe2++ Cl2= Fe3++Cl-ʵ��FcCl3��Һ�������𰸣�����������ۣ����� Cl2
��2����H2O2�����������¾���ǿ�����ԣ�������Cu����CuCl2��ʴ��ͭ����Ҫ��Ӧ�����ӷ���ʽΪCu + 2H++ H2O2![]() Cu2++ 2H2O��
Cu2++ 2H2O��
��HCl-H2O2��ʴ��Һ����Cu2�����ڼ�������������Cu(OH)2����������ԭ������Cu2O��ѡ������������Դ�㣬��������ʣ�������Cu2O�����У�������Լ�A�������ǡ�
������Cu2(OH)2CO3�Ĺ���������Ʒ�Ӧ���¶ȣ����¶ȸ���80��ʱ����Ʒ��ɫ��������ԭ������ǣ��¶ȸ���80��ʱ��Cu2(OH)2CO3�ֽ����ɺ�ɫCuO�����²�Ʒ��ɫ������
����ͼ�۲�֪20-30��ʱ�������¶�����ʴ��������������Ҫ����Ϊ�¶�����ʹ��Ӧ���ʼӿ���30�������������¶�����ʴ����������Ҫʱ��ΪH2O2�ֽ⡢NH3�ӷ���
(3)������������ӦH2O �C e�C![]() HO + H+��H++ Cu++ OH
HO + H+��H++ Cu++ OH![]() Cu2++ H2O����Cu2+��Cl��ͨ�������ӽ���Ĥ������������ CuCl2ʴ��Һ������
Cu2++ H2O����Cu2+��Cl��ͨ�������ӽ���Ĥ������������ CuCl2ʴ��Һ������