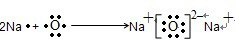
��Ŀ����
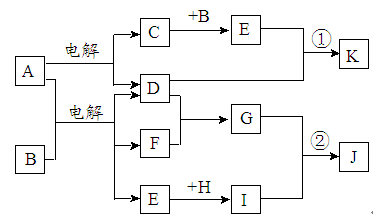
�ҹ����ٺ����ʡ�ݣ��ܹ�������ú�ˮ�Ƶö��ֻ�����Ʒ����ͼ���Ժ�ˮ�����ǣ���Ҫ�ɷ�CaCO3����Ϊԭ����ȡ���ֻ�����Ʒ�Ĺ�������ͼ������E��һ�ֻ��ʣ�N��һ�ֳ����Ľ������ʡ�

��������������̻ش��������⣺
��1������G��L�Ļ�ѧʽ�ֱ�Ϊ___________________��___________________��
��2���������������п���ѭ��ʹ�õ����ʵĻ�ѧʽΪ______________________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ____________���ڷ�Ӧ���б�����ͨ��NH3������ͨ��D����ԭ����_______________________________________________________��
��4����ҵ������F���Ƶ���һ�ֻ�����Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��5����K��Һ����δ��������Ƶ�N��_________________________________________________________��

��������������̻ش��������⣺
��1������G��L�Ļ�ѧʽ�ֱ�Ϊ___________________��___________________��
��2���������������п���ѭ��ʹ�õ����ʵĻ�ѧʽΪ______________________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ____________���ڷ�Ӧ���б�����ͨ��NH3������ͨ��D����ԭ����_______________________________________________________��
��4����ҵ������F���Ƶ���һ�ֻ�����Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��5����K��Һ����δ��������Ƶ�N��_________________________________________________________��
��1��CaCl2 H2 ��2��CO2
��3��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NH3����Һ�е��ܽ�ȴ���������CO2��ʹ��ת��ΪNH4HCO3
��4��2NaHCO3 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
��5��MgCl2��Һ�������Ȼ��������н��������ᾧ�Ƶ�MgCl2���壬���ڵ�����ȡ����þ
��3��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NH3����Һ�е��ܽ�ȴ���������CO2��ʹ��ת��ΪNH4HCO3
��4��2NaHCO3
 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O��5��MgCl2��Һ�������Ȼ��������н��������ᾧ�Ƶ�MgCl2���壬���ڵ�����ȡ����þ
�����Ӧע���������㣺
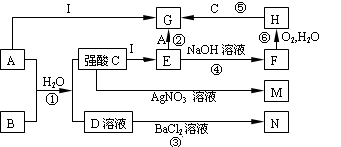
��1����ϵ�뺣ˮ�ۺ�������صĹ�ҵ����������������ͼ��
��2����ȷ��������ͼ�и���ĸ��Ӧ�����ʡ�
���еĹ�������ͼ�ɺ�ˮɹ�Ρ���ˮ��þ��ҵ�ƴ�����������ɡ�������������ͼ�е�ת����ϵ�ɵã�B��C��D��E��F��G��H��I��J��K��L��M��N�ֱ���NaCl��CaO��CO2��NH4Cl��NaHCO3��CaCl2��Mg��OH��2��MgO��HCl��MgCl2��H2��Cl2��Mg��
��1������G��L�Ļ�ѧʽ�ֱ�ΪCaCl2��H2��
��2�����ñ�������ʯ��ʱͬʱ����CO2���ڽ�NaHCO3���ȷֽ��ƴ���ʱҲ����CO2��CO2��ѭ�����á�
��3����Ӧ������NaCl��NH3��CO2��H2O��Ӧ��ȡNaHCO3��NH4Cl�ķ�Ӧ����ѧ����ʽΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��NH3��ˮ��Һ�е��ܽ�ȴ��������ս϶�����CO2ʹ��ת��ΪNH4HCO3��
��4����ҵ����NaHCO3���ȷֽ���Ƶô����ѧ����ʽΪ��2NaHCO3 Na2CO3+
Na2CO3+
CO2��+H2O��
��5��MgCl2��Һ�������Ȼ��������н��������ᾧ�Ƶ���ˮMgCl2���ٵ������MgCl2���Ƶý���þ��
��1����ϵ�뺣ˮ�ۺ�������صĹ�ҵ����������������ͼ��
��2����ȷ��������ͼ�и���ĸ��Ӧ�����ʡ�
���еĹ�������ͼ�ɺ�ˮɹ�Ρ���ˮ��þ��ҵ�ƴ�����������ɡ�������������ͼ�е�ת����ϵ�ɵã�B��C��D��E��F��G��H��I��J��K��L��M��N�ֱ���NaCl��CaO��CO2��NH4Cl��NaHCO3��CaCl2��Mg��OH��2��MgO��HCl��MgCl2��H2��Cl2��Mg��
��1������G��L�Ļ�ѧʽ�ֱ�ΪCaCl2��H2��
��2�����ñ�������ʯ��ʱͬʱ����CO2���ڽ�NaHCO3���ȷֽ��ƴ���ʱҲ����CO2��CO2��ѭ�����á�
��3����Ӧ������NaCl��NH3��CO2��H2O��Ӧ��ȡNaHCO3��NH4Cl�ķ�Ӧ����ѧ����ʽΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��NH3��ˮ��Һ�е��ܽ�ȴ��������ս϶�����CO2ʹ��ת��ΪNH4HCO3��
��4����ҵ����NaHCO3���ȷֽ���Ƶô����ѧ����ʽΪ��2NaHCO3
 Na2CO3+
Na2CO3+CO2��+H2O��
��5��MgCl2��Һ�������Ȼ��������н��������ᾧ�Ƶ���ˮMgCl2���ٵ������MgCl2���Ƶý���þ��

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
�����Ŀ