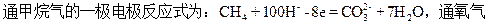��Ŀ����
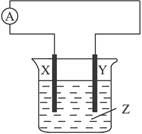
�������������������Զ�����ˮ�������ܽ��������������ڹ�ҵ��¯��ˮ��ʵ�����Ʊ�����ˮ�ȷ��档���������ǵĺ��IJ����������������������������������������������缫֮������һ�ȶ���ֱ����Դ�����Ȼ�����ҺΪ�������Һ���������Һ��ȡ��ˮ֮����һ�������������ı�Ĥ��������缫�ϲ����Ļ�ԭ�����Ĵ�С�ó������ĺ�����
�š���д�����������ǵ��йص缫��Ӧʽ
����_______________________________������____________________________________
�ơ������д�ĵ缫��Ӧʽ�Ʋ�Ӱ���������������ܵ����ؿ�������Щ��
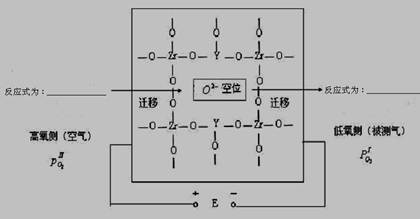
�ڸ����¸�¯���������IJⶨ���������(ZrO2)�����������ⶨ����������м���MgO,Y2O3��������ϵ�����������600�����ϸ���ʱ��Ϊ���Ŀ����ӵ��塣�䵼��������ҪԴ�ڲ��Ӿ����е������ӿ�λ����ͼΪ����ﯹ������ʵĵ���ԭ����
�ǡ�д��������͵�����ĵ缫��Ӧʽ
������______________________________��������________________________________
�ȡ���ij���ӵ缫Y2O3��ZrO2�����������ӿ�λ��Ϊ3.01��1020�����������ϲ��ӵ�Y2O3����Ϊ���ٿˣ�
�š���д�����������ǵ��йص缫��Ӧʽ
����_______________________________������____________________________________
�ơ������д�ĵ缫��Ӧʽ�Ʋ�Ӱ���������������ܵ����ؿ�������Щ��
�ڸ����¸�¯���������IJⶨ���������(ZrO2)�����������ⶨ����������м���MgO,Y2O3��������ϵ�����������600�����ϸ���ʱ��Ϊ���Ŀ����ӵ��塣�䵼��������ҪԴ�ڲ��Ӿ����е������ӿ�λ����ͼΪ����ﯹ������ʵĵ���ԭ����

�ǡ�д��������͵�����ĵ缫��Ӧʽ
������______________________________��������________________________________
�ȡ���ij���ӵ缫Y2O3��ZrO2�����������ӿ�λ��Ϊ3.01��1020�����������ϲ��ӵ�Y2O3����Ϊ���ٿˣ�
�š�������ӦO2 + 2H2O + 4e ="=" 4OH-
������ӦAg�C e- + Cl- ="=" AgCl ��2�֣���1�֣�
�ơ�pHֵ��H2S��Ũ�ȣ��ؽ������ӣ�д���������ɣ� ��2�֣�
�ǡ�O2+ 4e - ="==" 2O2- , 2O2-�� 4e- ="==" O2 ��2�֣���1�֣�
�ȡ�ÿ����1molY2O3�õ��������ӿ�λΪ1mol, �����Y2O3����Ϊ��
(3.01X1020/NA)´M Y2O3 =1��13´10-1g (��λ��Ч����)��2�֣�
������ӦAg�C e- + Cl- ="=" AgCl ��2�֣���1�֣�
�ơ�pHֵ��H2S��Ũ�ȣ��ؽ������ӣ�д���������ɣ� ��2�֣�
�ǡ�O2+ 4e - ="==" 2O2- , 2O2-�� 4e- ="==" O2 ��2�֣���1�֣�
�ȡ�ÿ����1molY2O3�õ��������ӿ�λΪ1mol, �����Y2O3����Ϊ��
(3.01X1020/NA)´M Y2O3 =1��13´10-1g (��λ��Ч����)��2�֣�
�Ŵ����У�һ���ؼ�������Ҫ�жϸõ绯ѧװ�õ����͡���Ȼ���и�����������װ���ǵ��أ����ǴӲ���ԭ���ȷ�����������ȴ��һ��ԭ���װ�á���װ������ԭ��ز����ĵ������ȶ�ֱ����ĵ������ã�����������ǿ�ȣ�����ȷ��O2��Ũ�ȡ���ˣ���������O2+ 2H2O + 4e ="=" 4OH-������O2��Ũ�Ȳ�һ����Ӱ���˸õ�Եĵ缫���ơ�����Agʧ���ӣ�����Cl���γɳ�����
��Ӱ���������������ؾ���Ӱ����������缫�缫���ƴ�С�����ء������ĵ�������ҺpH�йأ���������Ag(��)�Ĵ�����ʽ��Ӱ�쵽�õ缫�ĵ缫���ƣ����Ӱ��Ag(��)������ʽ�����ض�����������������ܲ���Ӱ�졣
�Ǹ������⣬�ù����������غ�����ΪO2-�������㶨������������һ�࣬�������࣬ʧȥ���ӣ�������ԭ��������˵缫��ӦʽΪ��O2+ 4e - ="==" 2O2- ����һ��O2-�õ�������O2��
�������ӿ�λ�����ʵ���Ϊ3.01��1020/6.02��1023=5.00��10-4(mol)��ÿһ��Y3+�û�һ��Zr4+������ɼ�С1����˸��ݵ���غ�ԭ��n(Y)=2n(Y2O3) =5.00��10-4��2 mol����n(Y2O3)=5.00��10-4 mol���Ӷ������Ħ��������
��Ӱ���������������ؾ���Ӱ����������缫�缫���ƴ�С�����ء������ĵ�������ҺpH�йأ���������Ag(��)�Ĵ�����ʽ��Ӱ�쵽�õ缫�ĵ缫���ƣ����Ӱ��Ag(��)������ʽ�����ض�����������������ܲ���Ӱ�졣
�Ǹ������⣬�ù����������غ�����ΪO2-�������㶨������������һ�࣬�������࣬ʧȥ���ӣ�������ԭ��������˵缫��ӦʽΪ��O2+ 4e - ="==" 2O2- ����һ��O2-�õ�������O2��
�������ӿ�λ�����ʵ���Ϊ3.01��1020/6.02��1023=5.00��10-4(mol)��ÿһ��Y3+�û�һ��Zr4+������ɼ�С1����˸��ݵ���غ�ԭ��n(Y)=2n(Y2O3) =5.00��10-4��2 mol����n(Y2O3)=5.00��10-4 mol���Ӷ������Ħ��������

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ
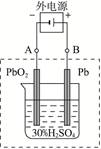
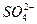
 2PbSO4+2H2O
2PbSO4+2H2O