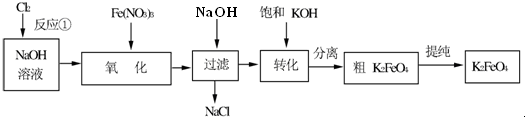
��Ŀ����
8����������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ���������̣�
�����������̻ش��������⣺
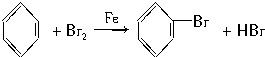
��1����Ϣ��з�����Ӧ�Ļ�ѧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4��
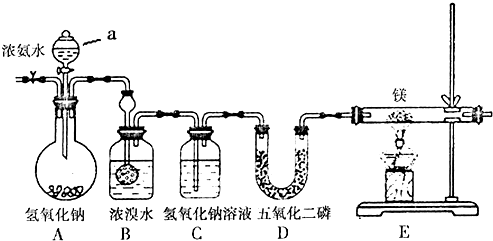
��2��������Ͳ���������Ʒֱ��ǹ��ˡ���������һ�������ڷ���d������ѡ���ţ�
a�������Һ�� b��������� c���������ܵ�Һ�� d�����ܵ�Һ��
��3����Ϣ��м���Na2SO3��Ŀ����SO2+Br2+2H2O=2HBr+H2SO4��
��4��������������ӦΪ��ɫҺ�壬��ʵ�ʹ�ҵ�������Ƶõ������ᣨ��ҵ�����ᣩ���е����Ļ�ɫ�����Ǽ�����ͬѧ����˼�ʵ�����̽������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3+��������֤���ü������õ��Լ�������ΪKSCN��Һ����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ����Br2��������֤���ü������õ��Լ��Ļ�ѧʽCCl4��
���� ��Ϣ��з�����Ӧ SO2+Br2+2H2O=H2SO4+2HBr��������������������������Ĵ�Ʒ�����壩����Ϣ��м���Na2SO3��ԭ��Ʒ�е�Br2�����ᷴӦ����SO42- ����������������˵����ᱵ��������ɫ��Һ��������õ����µ������ᣬ
��1��Br2����ǿ�����ԣ�����Һ�н�SO2����ΪH2SO4����������ԭΪHBr��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ����������һ�������ڷе㲻ͬ�Ļ��ܵ�Һ��ķ��룻
��3����Ʒ�п��ܺ���Ϊ��Ӧ��Br2��Ӧ��ȥBr2��
��4����KSCN��Һ����Fe3+���μ�KSCN��Һ����Һ���Ѫ��ɫ���ɹ������̿�֪����Һ�п��ܺ���Br2��������CCl4��ȡ�������飻
��� �⣺��Ϣ��з�����Ӧ SO2+Br2+2H2O=H2SO4+2HBr��������������������������Ĵ�Ʒ�����壩����Ϣ��м���Na2SO3��ԭ��Ʒ�е�Br2�����ᷴӦ����SO42- ����������������˵����ᱵ��������ɫ��Һ��������õ����µ������ᣬ
��1��Br2����ǿ�����ԣ�����Һ�н�SO2����ΪH2SO4����������ԭΪHBr����Ӧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4��
�ʴ�Ϊ��SO2+Br2+2H2O=2HBr+H2SO4��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ����������һ�������ڷе㲻ͬ�Ļ��ܵ�Һ��ķ��룬
�ʴ�Ϊ�����ˣ�����d��
��3����Ʒ�п��ܺ���Ϊ��Ӧ��Br2������Na2SO3����ȥ��Ʒ��δ��Ӧ����壬
�ʴ�Ϊ����ȥ��Ʒ��δ��Ӧ����壻
��4����KSCN��Һ����Fe3+��ȡ������Һ�μ�KSCN��Һ����Һ���Ѫ��ɫ��˵��������ʵ���ɫ����Ϊ��Fe3+���ɹ������̿�֪����Һ�п��ܺ���Br2��������CCl4��ȡ�������飬ȡ������Һ�������÷ֲ㣬�²�ʳȺ�ɫ��˵��������ʵ���ɫ����Ϊ��Br2��
�ʴ�Ϊ��KSCN��Һ������Br2��CCl4��
���� ���Ʊ�������Ϊ���壬����ѧ���Թ������̵����⡢���ʵķ����ᴿ�Ȼ������������Ӽ��顢�������ʵȣ��Ѷ��еȣ��Ƕ�֪ʶ���ۺ����ã���ѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�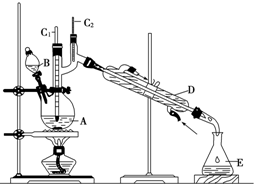 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.810 9 | �� |
| ����ȩ | 75.7 | 0.801 7 | �� |
��6.0g Na2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ��������ȴ�ӣ�
��2������װ��ͼ�У�D������������ֱ�������ܣ�
��3��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���£���ϡ����¡����㣮
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
A����ʪ���� B��������� C����©����D���궨��
| �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1 | n��N2�� | 0 | 0.20 | 0.35 | 0.40 | 0.40 |
| T2 | n��N2�� | 0 | 0.25 | �� | 0.30 | 0.30 |
| A�� | T1�¶��£�CH4��ƽ��ת����Ϊ50% | |
| B�� | T1��T2 | |
| C�� | a��0 | |
| D�� | T2ʱ��Ӧ��ƽ�ⳣ������T1ʱ��Ӧ��ƽ�ⳣ�� |
| A�� | 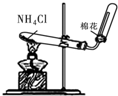 | B�� |  | C�� | 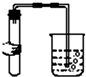 | D�� | 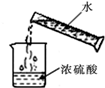 |
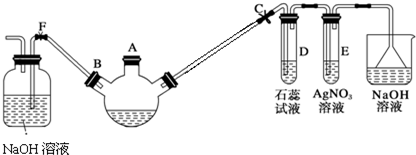
 ��
��