��Ŀ����
13��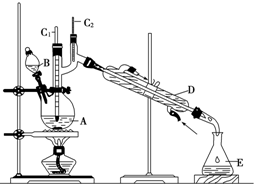 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.810 9 | �� |
| ����ȩ | 75.7 | 0.801 7 | �� |
��6.0g Na2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ��������ȴ�ӣ�
��2������װ��ͼ�У�D������������ֱ�������ܣ�
��3��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���£���ϡ����¡����㣮
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
A����ʪ���� B��������� C����©����D���궨��
���� ���������ô��Ĵ������Ʊ�����ȩ��ʵ������⣬��Ҫ�漰��Ӧ��ϻ�ϼ��ȷ����д�ʩ��Ӧ����������������������������������Լ��ֲ�Ʒ�ķ����ᴿ��
��1����ϼ���Ϊ��ֹ��ֹ����Ҫ�����ʯ�������Ⱥ���δ�ӷ�ʯ��Ӧ����ȴ�ӣ�
��2����������D������������������D����������ֱ�������ܣ�
��3���ɱ������ݿ�֪������ȩ�ܶ�С��ˮ���ܶȣ��ݴ��жϣ�
��4����Һ©��ʹ��ǰ������еĵ�һ�������Ǽ�©��
��� �⣺��1����ֹ����Ҫ�����ʯ�������Ⱥ���δ�ӷ�ʯ��Ӧ����ȴ�ӣ��ʴ�Ϊ����ֹ���У���ȴ�ӣ�
��2��D����������װ�ã�����Ϊֱ�������ܣ��ʴ�Ϊ��ֱ�������ܣ�
��3������ȩ�ܶ�Ϊ0.8017 g•cm-3��С��ˮ���ܶȣ������á��ֲ��ˮ�����·����ʴ�Ϊ���£�
��4����Һ©�����л�����ʹ��ǰ������еĵ�һ������Ǽ�©���ʴ�Ϊ��c��
���� ���⿼���л���ѧʵ����������ȣ��ѶȲ���ע��Ի���֪ʶ����������ͬʱ�������������Ϣ�������⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
1������������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ��������ʣ��й������Ʊ�����������ǣ�������

| A�� | �Ʊ���ʽ�����������˹�������������� | |
| B�� | Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��� | |
| C�� | ����KSCN��Һ���飨NH4��2Fe��SO4��2�Ƿ����� | |
| D�� | �Ʊ���NH4��2Fe��SO4��2�����������ܽ�ȱ�FeSO4���ܽ�ȴ���һ���� |
5��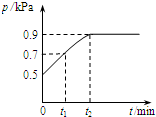 ��1.0L�ܱ������з���0.1mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0 ������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
��1.0L�ܱ������з���0.1mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0 ������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
 ��1.0L�ܱ������з���0.1mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0 ������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
��1.0L�ܱ������з���0.1mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0 ������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������| A�� | t1ʱn��X��=0.04 mol | |
| B�� | t1��t2����������ƽ����Է��������� | |
| C�� | �����ƽ����ϵ��Y�ĺ�������������ϵ�¶Ȼ����Z���� | |
| D�� | �����������䣬�ٳ���0.1 mol ����X��ƽ�������ƶ���X��ת���ʼ��� |
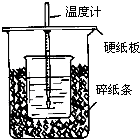 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺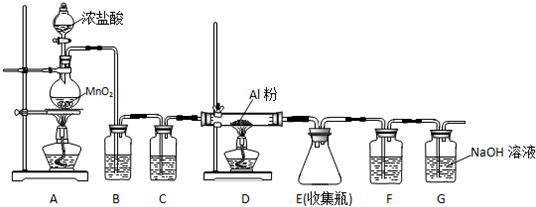

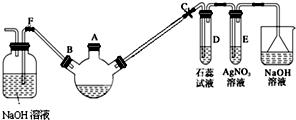 ij��ѧ������ȤС��ѧ������ͼ��ʾ��װ��̽������Һ��ķ�Ӧ���Ʊ��屽���������ش��������⣺
ij��ѧ������ȤС��ѧ������ͼ��ʾ��װ��̽������Һ��ķ�Ӧ���Ʊ��屽���������ش��������⣺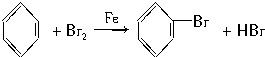 ��
��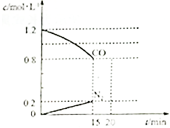 Ŀǰ���п���������ԭ��֮һ�ǻ�����β����ȼú������������NO��CO��Ϊ����β���ijɷ֣������������ڴ�ת�����з������·�Ӧ��
Ŀǰ���п���������ԭ��֮һ�ǻ�����β����ȼú������������NO��CO��Ϊ����β���ijɷ֣������������ڴ�ת�����з������·�Ӧ��
 ��
��