��Ŀ����
����Ŀ���±���Ԫ�����ڱ���ǰ�����ڣ�
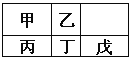
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0[ | |
һ | A | |||||||
�� | B | C | D | E | F | |||
��[ | G | H | I | J |
�ش��������⣺
��1��JԪ�ص�Ԫ�ص����ƣ�___________��
��2��GԪ����I Ԫ���γɵĻ�����ĵ���ʽ��___________��
A��G��E�γɵĻ������к��еĻ�ѧ������Ϊ_____________________________��
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����_____________��
�������������������_______________���û�����Ļ�ѧʽ��ʾ����
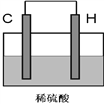
��4����H������C��һ�ֵ��ʣ����壩������ͼװ�����ӣ�����C��Ϊ_________��������������д���õ缫��ӦʽΪ��____________________��
���𰸡� � ![]() ���Ӽ������ۼ� HClO4 Al(OH)3 �� 2H����2e- = H2 ��
���Ӽ������ۼ� HClO4 Al(OH)3 �� 2H����2e- = H2 ��
������������Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪLi��CΪC��DΪN��EΪO��FΪF��GΪNa��HΪAl��IΪCl��JΪAr��
(1)JԪ�ص�����Ϊ벣��ʴ�Ϊ�����
(2)GԪ����I Ԫ���γɵĻ�����Ϊ�Ȼ��ƣ��������ӻ��������ʽΪ![]() ��A��G��E�γɵĻ�����Ϊ�������ƣ��������ӻ���������������к��й��ۼ������еĻ�ѧ�����������Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��A��G��E�γɵĻ�����Ϊ�������ƣ��������ӻ���������������к��й��ۼ������еĻ�ѧ�����������Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
(3)ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����HClO4���������������������Al(OH)3���ʴ�Ϊ��HClO4��Al(OH)3��
(4)���ܹ������ᷢ��������ԭ��Ӧ�������Ϊ������̼Ϊ�����������������ӵõ����������������缫��ӦʽΪ2H����2e- = H2 �����ʴ�Ϊ������2H����2e- = H2 ����