��Ŀ����
����Ŀ��[��ѧһѡ��3: ���ʽṹ������]
A��B��C��DΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ���ԭ��������������C��Dͬ���ڣ���A��B��C������ͬһ���ڣ�����A��Dͬ���壬�Ҹ������������ַǽ���Ԫ�أ�BΪ�ǽ���Ԫ����ԭ���������3�ԳɶԵ��ӣ�CԪ��λ��Ԫ�����ڱ���10�С���ش���������:
(1)CԪ�ص�ԭ������Ϊ________����̬Dԭ�ӵļ����Ų�ʽΪ________��
(2)��Aͬһ���ڵ���������Ԫ���е�һ������С��A��Ԫ�ع���_______�֡�
(3) DԪ�ؿ��γ�DX3��±������ʺͽṹ��AX3����(X��ʾ±��Ԫ��)����ˮ��Һ��ǿ��ˮ�⣬��д��DCl3��ˮ��Ӧ�Ļ�ѧ����ʽ:____________��
(4) ��ͼΪD2B3�ķ��ӽṹͼ��B�ӻ���ʽΪ____________��

(5) �ԱȽ�A��B�γɵļ��⻯����۷е�ĸߵͲ����ͣ�________ (���⻯���÷���ʽ��ʾ)��
(6) �о�����ṹ����÷�����________����ͼΪһ�ֺ�C��D����Ԫ����Ʒ��ľ���ͼ���������ṹ����������Ϊ����߳�Ϊanm����Ϊbmm��
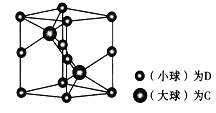
�ٸþ���������ʵĻ�ѧʽΪ__________��
����֪D��C�����ԭ�������ֱ�ΪM1��M2,�������ܶ�Ϊ��g/cm3,NA��ʾ�����ӵ��������ú�������ʽ��ʾ�þ�����ܶ�Ϊ________g/cm3 (�г�����ʽ���ɣ����ػ���)��
���𰸡� 28 [Ar]3d104s24p3 5 AsCl3+3H2O=H3AsO3+3HCl sp3�ӻ� NH3��HCl��NH3���ڷ��Ӽ������HCl���Ӽ�ֻ���ڷ��»���������������ȷ��»���ǿ��NH3���۷е����HCl X-�������� NiAs��AsNi 
��������������������⿼�����ʽṹ�����ʣ��漰Ԫ�ص��ƶϣ������Ų�ʽ����д����һ�����ܵıȽϣ��ӻ���ʽ���жϣ��۷е�ߵ͵ıȽϣ������ķ����ͼ��㡣C��ǰ������Ԫ�أ�CԪ��λ��Ԫ�����ڱ���10����CΪNiԪ����Ni���ڵ������ڣ�C��Dͬ���ڣ�DҲ���ڵ������ڣ�A��B��C��D��ԭ��������������A��B��C������ͬһ���ڣ�A��Dͬ�����Ҹ������������ַǽ���Ԫ�أ�AΪNԪ�أ�DΪAsԪ�أ�B���ڵ���������BΪ�ǽ�����ԭ���������3�ԳɶԵ��ӣ�BΪClԪ����
��1��CΪNiԪ����Ni��ԭ������Ϊ28��DΪAs��As��ԭ������Ϊ33�����ݹ���ԭ������̬As�ĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3�������Ų�ʽΪ[Ar] 3d104s24p3��
��2��AΪN��N���ڵڶ����ڣ��ڶ������е�һ������С��N��Ԫ����Li��Be��B��C��O����5����
��3��AsCl3��ˮ��Һ��ǿ��ˮ������H3AsO3��HCl����Ӧ�Ļ�ѧ����ʽΪAsCl3+3H3O=H3AsO3+3HCl��
��4����ʾ��ͼ�ɼ�B�γ�2���Ҽ���B�ϻ������Թµ��Ӷԣ�B�ļ۲���Ӷ���Ϊ4��B���ӻ���ʽΪsp3�ӻ���
��5��A�ļ��⻯��ΪNH3��B�ļ��⻯��ΪHCl������NH3���ڷ��Ӽ������HCl���Ӽ�ֻ���ڷ��»���������������ȷ��»���ǿ��NH3���۷е����HCl��
��6���о�����ṹ��õķ�����X-�������䡣
��a��������̯������DΪAs��8![]() +4
+4![]() =2��CΪNi��Cȫ�ھ����ڣ�Ni��2��Ni��As�ĸ�����Ϊ2:2=1:1���þ���Ļ�ѧʽΪNiAs��AsNi��
=2��CΪNi��Cȫ�ھ����ڣ�Ni��2��Ni��As�ĸ�����Ϊ2:2=1:1���þ���Ļ�ѧʽΪNiAs��AsNi��
��b���þ����������ṹ�������ĵ����Ϊa![]() 10-7cm
10-7cm![]() a
a![]() 10-7cm=
10-7cm=![]() a2
a2![]() 10-14cm2�����������Ϊ
10-14cm2�����������Ϊ![]() a2
a2![]() 10-14cm2
10-14cm2![]() b
b![]() 10-7cm=
10-7cm=![]() a2b
a2b![]() 10-21cm3��1mol��������Ϊ
10-21cm3��1mol��������Ϊ![]() a2b
a2b![]() 10-21cm3
10-21cm3![]() 2
2![]() NA������Ļ�ѧʽΪNiAs��AsNi��1mol���������Ϊ��M1+M2��g��������ܶ�=
NA������Ļ�ѧʽΪNiAs��AsNi��1mol���������Ϊ��M1+M2��g��������ܶ�=![]() =��M1+M2��g
=��M1+M2��g![]() ��
��![]() a2b
a2b![]() 10-21cm3
10-21cm3![]() 2
2![]() NA��=
NA��=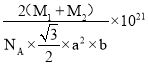 g/cm3��
g/cm3��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�����Ŀ�������������ڰ뵼���������ҵӦ�ù㷺��ijЩ���������к���CoԪ�أ��ӷ���(��Co3O4��Al2O3��Li2O��Fe2O3������) ���Ʊ��ߴ��ȵ������ܵ��������������
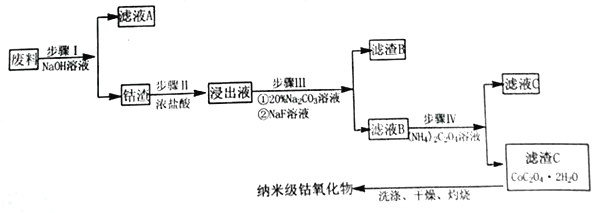
��֪:
��LiF ������ˮ��Li2CO3����ˮ��
����Ԫ�س����Ļ��ϼ�Ϊ+2 ��+3��
�۲��ֽ��������γ��������������pH���±���
Fe3+ | Co2+ | Co3+ | Al3+ | |
pH����ʼ������ | 1.9 | 7.15 | -0.23 | 3.4 |
pH����ȫ������ | 3.2 | 9.15 | 1.09 | 4.7 |
��1���������з��������ӷ�Ӧ����ʽ_______________��
��2������II��Ũ�����������_______________��
��3������III��Na2CO3��Һ�������ǵ�����Һ��pH,Ӧʹ��Һ��pH��ȡֵ��ΧΪ_______________�� ���� B ����Ҫ�ɷ�Ϊ_________________��
��4��������μ�����ҺB���Ƿ��в�����Fe3+:_______________��
��5�� ��������ϴ�ӡ��������أ�������Ϊ3.66gCoC2O4��2H2O�������գ���������������ͼ��ʾ��
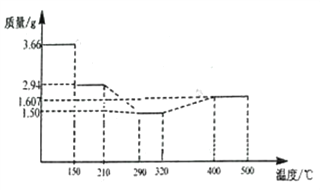
210��~290��ʱ�������������壬������Ӧ�Ļ�ѧ����ʽΪ_______________��400��~500�����ù���Ļ�ѧʽΪ_______________��