题目内容
【题目】实验室合成乙酸乙酯的步骤如下:在圆底烧瓶内加入乙醇、浓硫酸和乙酸,瓶口竖直安装通有冷却水的冷凝管(使反应混合物的蒸气冷凝为液体流回烧瓶内),加热回流一段时间后换成蒸馏装置进行蒸馏(如下图所示),得到含有乙醇、乙酸和水的乙酸乙酯粗产品。请回答下列问题:
(已知:乙醇、乙酸、乙酸乙酯的沸点依次是78.4℃、118℃、77.1℃)

(1)在烧瓶中除了加入乙醇、浓硫酸和乙酸外,还应放入_____,其目的是________________________。
(2)在烧瓶中加入一定比例的乙醇和浓硫酸的混合液的方法是:______________________。
(3)在该实验中,若用1 mol乙醇和1 mol乙酸在浓硫酸作用下加热,充分反应,能否生成1 mol乙酸乙酯,为什么?__________________________________________。
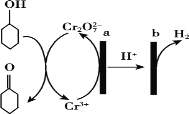
(4)现拟分离含乙酸、乙醇和水的乙酸乙酯粗产品,下图是分离操作步骤流程图。请在图中圆括号内填入适当的试剂,在方括号内填入适当的分离方法。
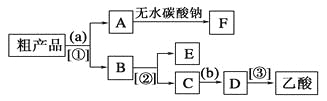
试剂a是__________,试剂b是__________;分离方法①是________,分离方法②是________,分离方法③是______。
(5)在得到的A中加入无水碳酸钠粉末,振荡,目的是________________________________。
【答案】碎瓷片 防止烧瓶中液体暴沸 先在烧瓶中加入一定量的乙醇,然后慢慢将浓硫酸加入烧瓶,边加边振荡 否,该反应是可逆反应,反应不能进行完全 饱和Na2CO3溶液 浓硫酸 分液 蒸馏 蒸馏 除去乙酸乙酯中混有的少量水
【解析】
(1)在烧瓶中除了加入乙醇、浓硫酸和乙酸外,还应放入碎瓷片,其目的是碎瓷片防止烧瓶中液体暴沸;
(2)溶液在混合时通常将浓度大的溶液注入到浓度小的溶液,故在烧瓶中加入一定比例的乙醇和浓硫酸的混合液的方法是:先在烧瓶中加入一定量的乙醇,然后慢慢将浓硫酸加入烧瓶,边加边振荡;
(3)1 mol乙醇和1 mol乙酸在浓硫酸作用下加热,充分反应,不能生成1 mol乙酸乙酯,因为该反应是可逆反应,反应不能进行完全;
(4)除去乙酸乙酯中的乙醇和乙酸用饱和Na2CO3溶液,溶液分层,用分液的方法分离,A是乙酸乙酯,B是乙酸钠和乙醇的混合溶液,用蒸馏的方法分离,E是乙醇,C是乙酸钠,要得到乙酸就加入强酸,所以试剂B是浓硫酸。根据分析,试剂a是饱和Na2CO3溶液,试剂b是浓硫酸;分离方法①是分液,分离方法②是蒸馏,分离方法③是蒸馏;
(5)得到的乙酸乙酯中还有少量的水,所以加入无水碳酸钠粉末,振荡,目的是除去乙酸乙酯中混有的少量水。

 开心快乐假期作业暑假作业西安出版社系列答案
开心快乐假期作业暑假作业西安出版社系列答案 名题训练系列答案
名题训练系列答案