题目内容
(4分)已知:C(s)+O2(g)  CO2(g) ΔH="-437.3" kJ·mol一1
CO2(g) ΔH="-437.3" kJ·mol一1
H2(g)+ O2(g)
O2(g)  H2O(g) ΔH ="-285.8" kJ·mol一1
H2O(g) ΔH ="-285.8" kJ·mol一1
CO(g)+ O2(g)
O2(g)  CO2(g) ΔH ="-283.0" kJ·mol一1
CO2(g) ΔH ="-283.0" kJ·mol一1
则煤的气化主要反应(碳和水蒸气反应生成一氧化碳和氢气)的热化学方程式是
 CO2(g) ΔH="-437.3" kJ·mol一1
CO2(g) ΔH="-437.3" kJ·mol一1H2(g)+
 O2(g)
O2(g)  H2O(g) ΔH ="-285.8" kJ·mol一1
H2O(g) ΔH ="-285.8" kJ·mol一1CO(g)+
 O2(g)
O2(g)  CO2(g) ΔH ="-283.0" kJ·mol一1
CO2(g) ΔH ="-283.0" kJ·mol一1则煤的气化主要反应(碳和水蒸气反应生成一氧化碳和氢气)的热化学方程式是
(4分)C(s) + H2O(g)  CO(g) + H2(g) ΔH=131.5 kJ·mol-1(热化学方程式状态漏写或反应热错均不得分)
CO(g) + H2(g) ΔH=131.5 kJ·mol-1(热化学方程式状态漏写或反应热错均不得分)
 CO(g) + H2(g) ΔH=131.5 kJ·mol-1(热化学方程式状态漏写或反应热错均不得分)
CO(g) + H2(g) ΔH=131.5 kJ·mol-1(热化学方程式状态漏写或反应热错均不得分)①C(s)+O2(g)  CO2(g) ΔH="-437.3" kJ·mol一1
CO2(g) ΔH="-437.3" kJ·mol一1
②H2(g)+ O2(g)
O2(g)  H2O(g) ΔH ="-285.8" kJ·mol一1
H2O(g) ΔH ="-285.8" kJ·mol一1
? CO(g)+ O2(g)
O2(g)  CO2(g) ΔH ="-283.0" kJ·mol一1
CO2(g) ΔH ="-283.0" kJ·mol一1
①+(-②)+(-?),得C(s) + H2O(g) CO(g) + H2(g) ΔH=+131.5 kJ·mol-1
CO(g) + H2(g) ΔH=+131.5 kJ·mol-1
点评:考查热化学方程式的相关计算;
 CO2(g) ΔH="-437.3" kJ·mol一1
CO2(g) ΔH="-437.3" kJ·mol一1②H2(g)+
 O2(g)
O2(g)  H2O(g) ΔH ="-285.8" kJ·mol一1
H2O(g) ΔH ="-285.8" kJ·mol一1? CO(g)+
 O2(g)
O2(g)  CO2(g) ΔH ="-283.0" kJ·mol一1
CO2(g) ΔH ="-283.0" kJ·mol一1①+(-②)+(-?),得C(s) + H2O(g)
 CO(g) + H2(g) ΔH=+131.5 kJ·mol-1
CO(g) + H2(g) ΔH=+131.5 kJ·mol-1点评:考查热化学方程式的相关计算;

练习册系列答案
 名校课堂系列答案
名校课堂系列答案
相关题目
 O2(g)
O2(g)  SO3(g) ΔH="-98.32" kJ·mol-1,在容器中充入 2 mol SO2和1 mol O2充分反应,最终放出的热量为( )
SO3(g) ΔH="-98.32" kJ·mol-1,在容器中充入 2 mol SO2和1 mol O2充分反应,最终放出的热量为( ) 。
。
 一是丁烷。在101 kPa时,10 kg丁烷(相对分子质量为58)完全燃烧生成CO2和液态H2O放出热量5×105 kJ,丁烷的燃烧热为___________,丁烷燃烧的热化学方程式为_________________________________________________。
一是丁烷。在101 kPa时,10 kg丁烷(相对分子质量为58)完全燃烧生成CO2和液态H2O放出热量5×105 kJ,丁烷的燃烧热为___________,丁烷燃烧的热化学方程式为_________________________________________________。 FeO(s) ?
FeO(s) ? 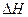 =-272.0KJ/mol
=-272.0KJ/mol 则 Al(s)的单质和FeO(s)反应的热化学方程式是_____________________________________。
则 Al(s)的单质和FeO(s)反应的热化学方程式是_____________________________________。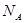 代表阿伏加德罗常数,则关于热化学方程式:C2H2(g) +
代表阿伏加德罗常数,则关于热化学方程式:C2H2(g) + 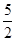 O2(g) ="===" 2 CO2(g) + H2O(l) △H="-1300" kJ/mol的说法中,正确的是( )
O2(g) ="===" 2 CO2(g) + H2O(l) △H="-1300" kJ/mol的说法中,正确的是( )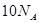 个电子转移时,该反应放出
个电子转移时,该反应放出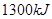 的能量
的能量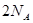 个碳氧共用电子对生成时,放出
个碳氧共用电子对生成时,放出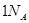 个水分子生成且为液体时,吸收
个水分子生成且为液体时,吸收