��Ŀ����
����Ŀ���ҹ������Ƽ���ʳƷҩƷ��CaԪ�غ����IJⶨ����֮һ������Na2C2O4�����������Ʒ�е�Ca2������������ϴ�ӣ�Ȼ������CaC2O4�������ڹ�����ǿ�ᣬ���ʹ����֪Ũ�ȵ�KMnO4��Һͨ���ζ����ⶨ��Һ��Ca2���ĺ�������Ը�ʵ���еĵζ����̣��ش��������⣺
(1)KMnO4��ҺӦ����________(������ʽ��������ʽ��)�ζ���ʢװ��
(2)д���ζ������з�Ӧ�����ӷ���ʽ��_____________��
(3)�ζ��յ����ɫ�仯����Һ��________ɫ��Ϊ________ɫ��
(4)������Щ�����ᵼ�²ⶨ�Ľ��ƫ��________(����ĸ���)��
a��װ��KMnO4��Һǰδ��ϴ�ζ���
b���ζ��������Ӷ���
c���ζ������ζ��ܼ������һ����Һ
d���ζ������У���ʱ������Һ����
(5)ijͬѧ������ʵ�鷽�������˸Ľ������ڲⶨijƷ�Ƶĸ�Ƭ�еĸ�Ԫ��(��ҪΪCaCO3)��������ʵ��������£�ȡ2.00 g��Ʒ������ƿ�У�����ʽ�ζ�������ƿ�ڼ���20.00 mLŨ��Ϊ0.10 mol��L��1������(�������)����ַ�Ӧһ��ʱ�䣬�þƾ��ƽ���ƿ��Һ����������ڣ������Ӻ���ȴ�����£�����2��3�����ָʾ������Ũ��Ϊ0.10 mol��L��1��NaOH��Һ�ζ����յ㣬����NaOH��Һ8.00 mL��[��ʾ��Ca(OH)2����ˮ��pH�ϵ�ʱ�������]
��Ϊʹ�������ԡ����ȷ���ζ������е����ָʾ��Ӧѡ��_______(����ʯ��������������������̪��)��Һ��
�ڴ�2.00 g��Ƭ��CaCO3������Ϊ________g��
���𰸡���ʽ 2MnO4-+5H2C2O4+6H=2Mn+10CO2��+8H2O �� �� ac ���� 0.06
��������
(1)������ؾ���ǿ�����ԣ��ḯʴ�������к͵ζ����������жϣ�
(2)�����������£�������ؽ�C2O42-����ΪCO2����������ԭΪMnSO4���ݴ�д����Ӧ�����ӷ���ʽ��
(3)���������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���뺬C2O42-��Һ��ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ���ݴ��жϣ�
(4)��������������V(��)��Ӱ�죬����c(����)=![]() �ж϶Խ����Ӱ�죻
�ж϶Խ����Ӱ�죻
(5)̼��ƺ����ᷴӦ�����Ȼ��ơ�������̼��ˮ�����Ƕ�����̼����ˮ����Ҫ������У������������������Ƶζ��������������Ƶ����ԣ�Ӧѡ����pH�ϵ�ʱ��ɫ��ָʾ�������ݵζ�����������ᣬ���������̼��Ʒ�Ӧ�����ᣬ���ݷ���ʽ����̼��Ƶ�������
(1)������ؾ���ǿ�����ԣ��ḯʴ��Ӧ������ʽ�ζ����У��ʴ�Ϊ����ʽ��
(2)������ؾ���ǿ�����ԣ������������½�C2O42-����ΪCO2����������ԭΪMn2+�����ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O���ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
(3)���������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ����C2O42-��ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ���ޣ��ϣ�
(4)a���ζ�ʱ���ζ��ܾ�ˮϴ������ˮϴ�����ζ������������Һ����ɱ�Һ�屻ϡ�ͣ�Ũ�ȱ�ϡ������V(��)ƫ����c(����)=![]() ��֪�����ƫ�ߣ���a��ȷ��b���ζ�ǰƽ�ӣ��ζ����ӣ������ñ�Һ����ƫС������c(����)=
��֪�����ƫ�ߣ���a��ȷ��b���ζ�ǰƽ�ӣ��ζ����ӣ������ñ�Һ����ƫС������c(����)=![]() ��֪�����ƫ�ͣ���b����c���ζ������ζ��ܼ������һ����Һ�����V(��)ƫ����c(����)=
��֪�����ƫ�ͣ���b����c���ζ������ζ��ܼ������һ����Һ�����V(��)ƫ����c(����)=![]() ��֪�����ƫ�ߣ���c��ȷ��d���ζ������У���ʱ������Һ���������ñ�ҺV(��)ƫС������c(����)=
��֪�����ƫ�ߣ���c��ȷ��d���ζ������У���ʱ������Һ���������ñ�ҺV(��)ƫС������c(����)=![]() ��֪�����ƫ�ͣ���d���ʴ�Ϊ��ac��
��֪�����ƫ�ͣ���d���ʴ�Ϊ��ac��
(5)�ٸ��������Ϣ��Ca(OH)2����ˮ��pH�ϵ�ʱ��������������ȱ�ɫ��ΧΪ3.1��4.4�����ϣ�ʯ���ɫ�����ԣ���̪��ɫ��ΧΪ��8.2��10.0��pH�ϸߣ����������Ƴ������ɣ����ŵζ������ü�����ָʾ�����ʴ�Ϊ�����ȣ�
���к͵ζ�������������ʵ���=�������Ƶ����ʵ���=0.1mol/L��8mL=0.8mmol�����̼��Ʒ�Ӧ������Ϊ��0.1mol/L��20mL-0.8mmol=1.2mmol=0.0012mol��
CaCO3 + 2HCl=CaCl2+H2O+CO2��
100g 2mol
m 0.0012mol
m=![]() =0.06g���ʴ�Ϊ��0.06��
=0.06g���ʴ�Ϊ��0.06��

����Ŀ��H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
��.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ________��
(3)��֪��25 ��ʱ��Ksp(SnS)��1.0��10��25��Ksp(CdS)��8.0��10��27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2����ʼ����ʱ����Һ��c(Cd2��)��________(��Һ����仯���Բ���)��
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)��H2(g) ![]() H2S(g)��CO(g)����H����7 kJ��mol��1��
H2S(g)��CO(g)����H����7 kJ��mol��1��
��.CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H����42 kJ��mol��1��
CO2(g)��H2(g)����H����42 kJ��mol��1��
(4)��֪������1 mol�����еĻ�ѧ���������յ����������ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/(kJ��mol��1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
����x��________��
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g)����������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶�(T)�Ĺ�ϵ��ͼ��ʾ��
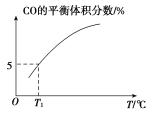
�������¶����ߣ�CO��ƽ���������_____(����������������С��)��ԭ��Ϊ_______
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶��£�COS��ƽ��ת����Ϊ_____����Ӧ����ƽ�ⳣ��Ϊ_____(������λ��Ч����)��
����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HC1O |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10 K2=5.6��10-11 | 3.0��10-8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HC1O��������ǿ������˳��Ϊ______________________��
��2��д��H2CO3�ĵ��뷽��ʽ��______________________��
��3��������0.1 mol��L-1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����______________________������ĸ��ţ���ͬ����
A.c(H+) B.c(H+)/c(CH3COOH)
C. c(H+)��c(OH-) D. 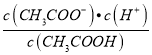
������Һ���{�¶ȣ�����4�ֱ���ʽ�������������_________________________��
��4��ȡ0.10mol CH3COOH �������ᣩ��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ���Ƚ�a��b���������ʣ��>����<����=������
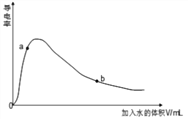
n(H+)��a_____b��c(CH3COO-)��a_____b����ȫ�к�ʱ����NaOH�����ʵ�����a_____b��
��5��H+Ũ����ͬ�������������ҺA(���ᣩ��B(CH3COOH)�քe��п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ�� ������˵����ȷ����__________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��B>A �ڿ�ʼ��Ӧʱ������A>B
�۲μӷ�Ӧ��п�����ʵ���A=B ��A����пʣ��
����Ŀ���±�ΪԪ�����ڱ���һ���֣���Ԫ�ط��Ż�ѧʽ�ش��������⡣
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� |
��1��д�������Ԫ����ɵ���ķ��ӵĵ���ʽ��________��
��2���ٵ���̬�⻯����������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ_____�����������г���Ԫ���������Ӱ뾶��С����______�������ӷ��ţ���
��3���ڢۢݼ���Ԫ������������Ӧ��ˮ���������ǿ����_______ (�ѧʽ)��Ԫ�آߵļ��⻯��ĽṹʽΪ___________�����⻯���Ԫ�آܵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ_______________________��
��4�������������ͬ�����Ԫ�صĵ��ʢߵĵ�����ȼ�����ɵĻ�����ĵ���ʽ________���ܢ��Ԫ�ص����ӵĻ�ԭ����ǿ����˳��Ϊ____________�������ӷ��ţ���