��Ŀ����
����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HC1O |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10 K2=5.6��10-11 | 3.0��10-8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HC1O��������ǿ������˳��Ϊ______________________��
��2��д��H2CO3�ĵ��뷽��ʽ��______________________��
��3��������0.1 mol��L-1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����______________________������ĸ��ţ���ͬ����
A.c(H+) B.c(H+)/c(CH3COOH)
C. c(H+)��c(OH-) D. 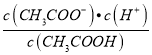
������Һ���{�¶ȣ�����4�ֱ���ʽ�������������_________________________��
��4��ȡ0.10mol CH3COOH �������ᣩ��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ���Ƚ�a��b���������ʣ��>����<����=������
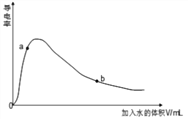
n(H+)��a_____b��c(CH3COO-)��a_____b����ȫ�к�ʱ����NaOH�����ʵ�����a_____b��
��5��H+Ũ����ͬ�������������ҺA(���ᣩ��B(CH3COOH)�քe��п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ�� ������˵����ȷ����__________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��B>A �ڿ�ʼ��Ӧʱ������A>B
�۲μӷ�Ӧ��п�����ʵ���A=B ��A����пʣ��
���𰸡� CH3COOH>H2CO3>HC1O H2CO3![]() HCO3-+H+ A ABCD < > = �ۢ�
HCO3-+H+ A ABCD < > = �ۢ�
����������1�������̶�Խ��������ͬ�¶��µĵ���ƽ�ⳣ����Խ���ݱ������ݿ�֪����ƽ�ⳣ��CH3COOH��H2CO3��HClO���������ԣ�CH3COOH��H2CO3��HClO����2��̼���Ƕ�Ԫ���ᣬ���뷽��ʽΪH2CO3![]() HCO3-+H+����3���ڳ����£���������Һ�д��ڵ���ƽ�⣺CH3COOH
HCO3-+H+����3���ڳ����£���������Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO-+H+��A��ϡ�ʹٽ����룬�����ӵ����ʵ������ӣ���������Ũ�ȼ�С��A��ȷ��B����Һ�ڼ�ˮϡ�����У�c(CH3COOH)��c(H+)����ϡ�Ͷ���С�����д�������ʵ�����С�������ӵ����ʵ������ӣ�������Һ��
CH3COO-+H+��A��ϡ�ʹٽ����룬�����ӵ����ʵ������ӣ���������Ũ�ȼ�С��A��ȷ��B����Һ�ڼ�ˮϡ�����У�c(CH3COOH)��c(H+)����ϡ�Ͷ���С�����д�������ʵ�����С�������ӵ����ʵ������ӣ�������Һ��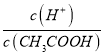 ���ߣ� B����C���¶Ȳ��䣬ˮ�����ӻ��������䣬C����D��
���ߣ� B����C���¶Ȳ��䣬ˮ�����ӻ��������䣬C����D��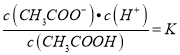 ���¶Ȳ��䣬���볣�����䣬D����ѡA������Һ���{�¶ȣ��ٽ����룬���볣��������Һ��������Ũ��������Ũ�ȼ�С����
���¶Ȳ��䣬���볣�����䣬D����ѡA������Һ���{�¶ȣ��ٽ����룬���볣��������Һ��������Ũ��������Ũ�ȼ�С����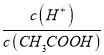 ���������¶�ˮ�����ӻ���������ѡABCD����4��ϡ�ʹٽ����룬��n(H+)��a��b������Ũ��Խ������Խǿ����c(CH3COO-)��a��b��a��b�����������ʵ�����ȣ������ȫ�к�ʱ����NaOH�����ʵ�����a��b����5��������Ũ����ͬ�ĵ������A��B������Һ��AΪ���ᣬBΪ���ᣩ�ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ�����ڴ��Ჿ�ֵ��룬�����Ũ�ȴ���������Ũ�ȣ�������HCl��Ũ�ȵ��������ӵ�Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������п��ʣ�ࣻ�����ڴ��������������Ӧ�Ͽ죬���Է�Ӧ�����ʱ��A��B�����ڿ�ʼpH��ͬ����������Ũ����ͬ�����Կ�ʼʱ��Ӧ����A=B�������������ɵ����������ͬ�����Բμӷ�Ӧ��п�����ʵ���A=B����ȷ���ܴ����Ũ�ȴ��������Ũ�ȣ�������ʣ�࣬����������п��ʣ�࣬��ȷ����ѡ�ۢ���
���������¶�ˮ�����ӻ���������ѡABCD����4��ϡ�ʹٽ����룬��n(H+)��a��b������Ũ��Խ������Խǿ����c(CH3COO-)��a��b��a��b�����������ʵ�����ȣ������ȫ�к�ʱ����NaOH�����ʵ�����a��b����5��������Ũ����ͬ�ĵ������A��B������Һ��AΪ���ᣬBΪ���ᣩ�ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ�����ڴ��Ჿ�ֵ��룬�����Ũ�ȴ���������Ũ�ȣ�������HCl��Ũ�ȵ��������ӵ�Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������п��ʣ�ࣻ�����ڴ��������������Ӧ�Ͽ죬���Է�Ӧ�����ʱ��A��B�����ڿ�ʼpH��ͬ����������Ũ����ͬ�����Կ�ʼʱ��Ӧ����A=B�������������ɵ����������ͬ�����Բμӷ�Ӧ��п�����ʵ���A=B����ȷ���ܴ����Ũ�ȴ��������Ũ�ȣ�������ʣ�࣬����������п��ʣ�࣬��ȷ����ѡ�ۢ���