��Ŀ����
����Ŀ�����ϱ�����MnO2��Fe3+��H2O2�Ʊ�O2�����д����á�ij����С��ͬѧ��ʵ���ҽ�������֤���鲢�о���H2O2�������ʡ��ش��������⣺
��1��������֧�ֱ�ʢ��2 mL10% H2O2��Һ���Թܣ���ͬѧ�ڵ�һ���ڶ�֧�Թ��зֱ����MnO2������FeCl3��Һ�����־��д����������ɣ������֧�Թ��м���������������Һ���۲�������Ŀ����________��
��2����ͬѧ����������ϣ���ô���������ͼ��ʾ��
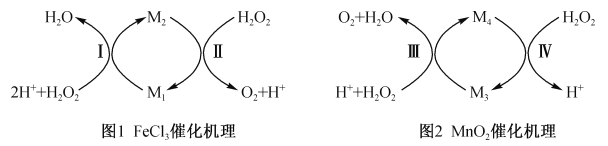
��ͼ1����M1��_______���ѧʽ����
��ͼ2����M4ΪMn2+����Ӧ�������ӷ���ʽΪ_____________��
��3����ͬѧ���Թ��м���2 ml. 3%��H2O2��Һ��0.5mL���ѣ�l mL1 mol/LH2SO4��Һ��3��4��0.5mol/L��K2Cr2O7��Һ�������ϲ��л���Ϊ��ɫ��CrO5��������Һ�����²�ˮ��Ϊ��ɫ��
�����ѳ���ΪCrO5���ȶ����⣬��һ������____________��
��һ��ʱ�����ɫ��ȥ������������������ͬʱˮ������Һ��Ϊ��ɫ����Cr3+������ɫ��Ӧ�����ӷ���ʽΪ________________��
���𰸡� ��֤FeCl3��Һ��ֻ��Fe3+���д����ã���Cl-û�� Fe2+ MnO2 +H2O2 +2 H+ =Mn2+ +O2��+2 H2O ��ȡCrO5 4CrO5 +12 H+=4Cr3++ 7O2��+6 H2O
����������1����Ϊ�Ȼ�����Һ���������Ӻ������ӣ����ݿ��Ʊ���������˵�����ʵ����Ϊ�Ա�ʵ�飬��Ŀ������֤FeCl3��Һ��ֻ��Fe3+���д����ã���Cl-û�С�
��2������ͼ1��֪��M1���Ա�˫��ˮ����ΪM2��M2����˫��ˮ����Ϊ����������M1���л�ԭ�ԡ�M2���������ԣ�����ͼ1����M1��Fe2+��
��ͼ2����M4ΪMn2+����Ӧ������ӷ���ʽΪMnO2 +H2O2 +2 H+ =Mn2+ +O2��+2 H2O��
��3����ͬѧ���Թ��м���2 ml. 3%��H2O2��Һ��0.5mL���ѣ�l mL1 mol/LH2SO4��Һ��3��4��0.5mol/L��K2Cr2O7��Һ�������ϲ��л���Ϊ��ɫ��CrO5��������Һ�����²�ˮ��Ϊ��ɫ��
�����ѳ���ΪCrO5���ȶ����⣬��һ����������ȡ������ȡCrO5 ��
��һ��ʱ�����ɫ��ȥ������������������ͬʱˮ������Һ��Ϊ��ɫ����Cr3+�������ݸ��Ļ��ϼ۽��ͣ������жϻ��ϼ����ߵ�Ԫ�����������Բ���������������������ɫ��Ӧ�����ӷ���ʽΪ4CrO5 +12 H+=4Cr3++ 7O2��+6 H2O��

����Ŀ��ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ������ѻ���ɱ�״̬����
ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
���������mL�� | 50 | 120 | 232 | 290 | 310 |
��1����һʱ��Σ�ָ0��1��1��2��2��3��3��4��4��5min����Ӧ�������
��2����һ��ʱ�εķ�Ӧ������С ��
��3����2��3����ʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ���ʣ�����Һ������䣩 ��
��4�������Ӧ̫���ң�Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ������������������������зֱ����������������Һ��
A.����ˮ
B.NaCl��Һ
C.Na2CO3��Һ
D.CuSO4��Һ
����Ϊ���е��ǣ����ţ� ��