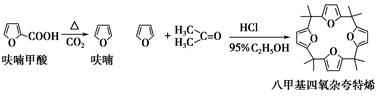
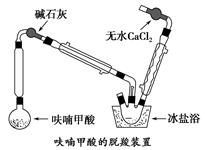
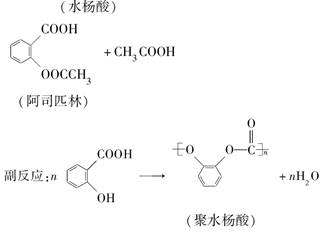
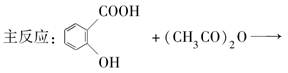��Ŀ����
ij��ȤС��̽��SO2���廹ԭFe3����I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��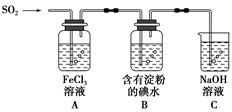
(1)SO2���廹ԭFe3���IJ�����________(�����ӷ���)���μӷ�Ӧ��SO2��Fe3�������ʵ���֮����________��
(2)����ʵ�鷽����������ʵ������ȡ����SO2����________��
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D��ͭ����ŨH2SO4
(3)װ��C��������____________________________________��
(4)��Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������________(�����)��
A���������� B��ʯ����
C��©�� D���ձ� E�������� F������
(5)������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3��������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ�м�����ϡ�����ữ��BaCl2��������ɫ������
������������������________��ԭ����__________________________
______________________________________________��
(6)�ܱ���I����ԭ������SO2��������_____________________________
___________________________________________��
��(1)Fe2����SO42����1��2��(2)BD��(3)���ն����SO2����ֹ��Ⱦ������(4)BF��(5)�����١�SO2Ҳ��ʹ����KMnO4��Һ��ɫ��(6)B����Һ��ɫ�䵭������ʧ
����

 ���б�ˢ��ϵ�д�
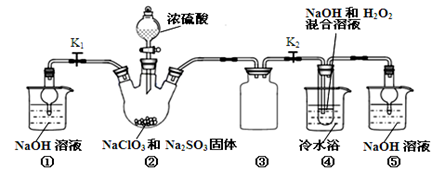
���б�ˢ��ϵ�д�ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է������������
| ���� | �״� | ������ | ��������� |
| �е㣯�� | 64.7 | 249 | 199.6 |
| ��Է������� | 32 | 122 | 136 |
��.�ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g�������20mL �״����ܶ�Լ0.79g/mL�� ����С�ļ���3mL Ũ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1���÷�Ӧ��Ũ��������� ������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ�� ���״�������ԭ�� ��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������ ��
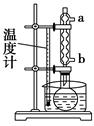
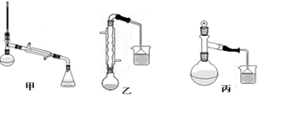
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ����ʵ������ȡ�����������װ�ã��г������ͼ�������������ȥ���������л�����ص㣬��ò��� װ�ã���ס������ҡ�������������
�ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ���������������ͼ���о��ƣ����������ͼ����ǡ���������������ƣ�����IΪ ������IIΪ ��
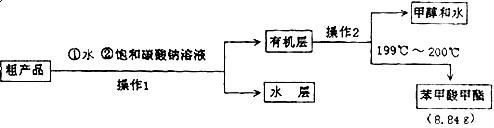
��5����������ͼ�м���Na2CO3��Һ�����Һ©���������ã�Ҫ�õ��л��㣬���������� ��
��6������������IJ���Ϊ ��
SiO2��SO2��CO2����������������ǵĻ�ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ�������ԡ�
ij��ȤС������ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣
(1)ѡ����ȡSO2�ĺ����Լ�________(����)��
��ŨHCl����ŨH2SO4����Na2SO3���塡��CaSO3����
(2)����װ�û����Ż����Ż��ķ�����________________________________________��װ��C��NaOH��Һ��������___________________________________________________________
(3)��ͬѧ�Ʋ�Mg��SO2�ķ�Ӧ��Mg��CO2�ķ�Ӧ���ƣ���÷�Ӧ����ʽΪ_________________________________________��
��ͬѧ���Ʋ��ǣ�2Mg��3SO2 2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2
2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2 2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
��֪��MgSO3��MgS������ˮ���������ᷢ�����ֽⷴӦ�ų����壻H2S����ͨ��CuSO4��Һ�г��ֺ�ɫ������
��ѡ�Լ���2 mol��L��1���ᡢ2 mol��L��1���ᡢ����ˮ��2 mol��L��1 NaOH��Һ��Ʒ����Һ������ʯ��ˮ��2 mol��L��1 CuSO4��Һ����������Ʒ��ѡ��
| ��� | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ȡ������Ӧ�����ù������Թ��� | |
| �� | ���Թ��еĹ��������μ�____________���Թܿ����ϴ����ܵĵ���������������ͨ��ʢ��________���Թ��� | ���Թ��е�________�����ͬѧ�Ʋ���ȷ�����Թ��еĹ���δ��ȫ�ܽ⣬��________������ͬѧ�Ʋ���ȷ |
��������ʵ��̽������֤����ͬѧ�Ʋ���ȷ�IJ�����Ԥ��������
_____________________________________________________________��
(4)����ʵ����Ҫ100 mL 2 mol��L��1�����ᣬ����ʱѡ��________(ѡ��10 mL��25 mL��50 mL��100 mL)��Ͳ��ȡ36.5%�ܶ�Ϊ1.19 g��mL��1��Ũ��������Ϊ________mL��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�
��1��ָ�������۵����ƣ� ���������г�������Cl2��Ŀ���� ��
��2����ȡ��Ĺ����У��ɹ�ѡ����Լ��� ( )
| A���ƾ� | B�����Ȼ�̼ | C������ | D������ |

��3���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ����辭������ͼI��ijͬѧ��Ƶ�����װ�ã�ͼ�����ԵĴ����� ��
��4����ͬѧ��Ϊ����ʱ���ʹ��ˮԡ���ȣ�ʹ��ˮԡ���ŵ��ǣ� ����������������ۼ��� �����������ƣ��
��5�� ʵ��۷�������ķ�Һ�к���Cl�C��SO42�C����ֻȡһ����Һ����μ����Cl�C��SO42�C�����μ����Լ���Ϊ�� �� ��
��6��ijС��ͬѧʵ��ʱ���õ�һ�����ʵ���Ũ�ȵĵ�ˮ��Һ225mL������ʱ��Ҫ����������ƽ�����������ձ��⣬���� �� ����ҡ��ʱ������Һ����ڿ̶��ߣ��������ҺŨ������ƫ��ƫС����Ӱ�죩��