��Ŀ����
1����1����״����9.6g��ij���壬�����0.6g���������ͬ�����������Է���������32����2����ͬ��ͬѹ�£�ij��������������Ϊ2��1��������Ϊ8��5���������������Է�������֮��Ϊ4��5��
��3����״����15g CO��CO2�Ļ����������Ϊ10.08L����˻�������ƽ��Ħ��������33.3g/mol�����������CO2�����ʵ�����0.15mol��CO��������8.4g��
���� ��1����ͬ�����¸����������������ͬ����������ʵ�����ȣ�����n=$\frac{m}{M}$�����������ʵ������ٸ���M=$\frac{m}{n}$������������Է���������
��2��ͬ��ͬѹ�£��������֮�ȵ��������ʵ���֮�ȣ��ٽ��M=$\frac{m}{n}$���������Է�������֮�ȣ�
��3������n=$\frac{V}{{V}_{m}}$���������������ʵ���������n=$\frac{m}{M}$����������ƽ��Ħ������������������CO��CO2�����ʵ����ֱ�Ϊxmol��ymol�������������������ʵ����з��̼�����
��� �⣺��1���������ʵ���Ϊ$\frac{0.6g}{2g/mol}$=0.3mol����ͬ�����¸����������������ͬ����������ʵ�����ȣ��ʸ��������Է�������Ϊ$\frac{9.6}{0.3}$=32���ʴ�Ϊ��32��
��2��ͬ��ͬѹ�£�ij��������������Ϊ2��1����������ʵ���֮��Ϊ2��1������������Ϊ8��5���������Է�������֮��Ϊ$\frac{8}{2}$��$\frac{5}{1}$=4��5���ʴ�Ϊ��4��5��
��3��������������ʵ���Ϊ$\frac{10.08L}{22.4L/mol}$=0.45mol���������ƽ��Ħ������Ϊ$\frac{15g}{0.45mol}$=33.3g/mol������������CO��CO2�����ʵ����ֱ�Ϊxmol��ymol����
$\left\{\begin{array}{l}{x+y=0.45}\\{28x+44y=15}\end{array}\right.$
���x=0.3��y=0.15
��CO������Ϊ0.3mol��28g/mol=8.4g��
�ʴ�Ϊ��33.3g/mol��0.15mol��8.4g��
���� ���⿼�����ʵ����йؼ��㣬ע��Թ�ʽ�����������Ӧ�ã������ڻ���֪ʶ�Ĺ��̣�

 ��У����ϵ�д�
��У����ϵ�д�������нϸ��۵�Ľ�������
����ڻ������г�+1��
��﮿�����ˮ��Ӧ�������ۻ���С��
��﮽����Ժܻ��ã���Ҫ������ú����
�ݼص�����ˮ��Ӧ���ң�����ˮ�����¸�����
| A�� | �ڢۢ� | B�� | �٢ۢ� | C�� | �٢ڢ� | D�� | �٢ܢ� |
 ��ͼ��ʾ��ֱ��ʯӢ��������г���CO���壬��˷��ò���������Ni���ۣ���һ�������£�Ni������CO��g���������·�Ӧ��Ni��s��+4CO��g��$?_{453��473K}^{323��353K}$Ni��CO��4��g��
��ͼ��ʾ��ֱ��ʯӢ��������г���CO���壬��˷��ò���������Ni���ۣ���һ�������£�Ni������CO��g���������·�Ӧ��Ni��s��+4CO��g��$?_{453��473K}^{323��353K}$Ni��CO��4��g����Ni���е����ʲ���CO��g��������Ӧ�����������������˵��¶ȷֱ��ȶ���350K��470K�������㹻��ʱ�������˵����ȷ���ǣ�������
| A�� | ��ʯӢ���Ҷ���Ҫ������Ni��CO��4��g�� | |
| B�� | ��ʯӢ���Ҷ���Ҫ�����Ǵ�Ni��s����CO��g�� | |
| C�� | ��ʯӢ�������Ҫ�����Ǵ�Ni��s����CO��g�� | |
| D�� | �������̿��Կ���CO��g����Ni��s����ʯӢ�����ת�Ƶ��Ҷˣ��Ӷ��ﵽ�ᴿĿ�� |
| A�� | NH3 | B�� | SO2 | C�� | CH4 | D�� | H2 |
| A�� | C3H6 | B�� | CH2O2 | C�� | C2H6O | D�� | C3H6O2 |


 ��ͼ��ʾˮ�����Թ�����һö��������������۲죺
��ͼ��ʾˮ�����Թ�����һö��������������۲죺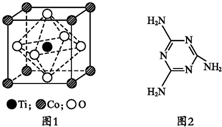 MnO2�Ǽ��̵�ز���������ͨ����������֮һ���ڻ��Բ���MnO2�м���CoTiO3�����壬��������������ʣ��Ż����̵�ص����ܣ�
MnO2�Ǽ��̵�ز���������ͨ����������֮һ���ڻ��Բ���MnO2�м���CoTiO3�����壬��������������ʣ��Ż����̵�ص����ܣ�