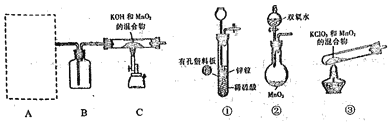
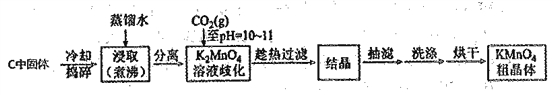
��Ŀ����
����Ŀ�������е��л�������ḻ������ʳס�еȶ��Ӧ�ù㷺�������Ҵ��������DZȽϳ������л��
(1)��ҵ������ϩ��ˮ��Ӧ���Ƶ��Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪ_______________(����д��Ӧ����)��
(2)�Ҵ��ܹ�����������Ӧ���Ҵ���ͭ�������������¿ɱ���������Ϊ��ȩ����Ӧ�Ļ�ѧ����ʽΪ_______��
(3)���й����Ҵ���˵����ȷ����______(ѡ����ĸ)��
A���Ҵ����ܺ����Ը��������Һ����������ԭ��Ӧ
B���Ҵ�ֻ�ܱ���������ȩ
C���ƾ���ijЩ����ʹ�Ҵ�����Ϊ���ᣬ���Ǿƾͱ�����
(4)����������е�O����18Oʱ���Ҵ������е�O����16Oʱ��������һ�������·�Ӧ����������ˮ����Է�������Ϊ________��
���𰸡�CH2=CH2��H2O![]() CH3CH2OH2CH3CH2OH��O2
CH3CH2OH2CH3CH2OH��O2![]() 2CH3CHO��2H2OC20
2CH3CHO��2H2OC20
��������
��1����ϩ��ˮ��Ӧ���Ƶ��Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪCH2=CH2��H2O![]() CH3CH2OH����2���Ҵ��ܹ�����������Ӧ���Ҵ���ͭ�������������¿ɱ���������Ϊ��ȩ��ͬʱ����ˮ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2
CH3CH2OH����2���Ҵ��ܹ�����������Ӧ���Ҵ���ͭ�������������¿ɱ���������Ϊ��ȩ��ͬʱ����ˮ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O��(3)A����CH2OHԭ���ſɱ�����KMnO4��Һ������ѡ��A����B���Ҵ�Ҳ��ֱ��������CH3COOH��ѡ��B����C���ƾ���ijЩ����ʹ�Ҵ�����Ϊ���ᣬ���Ǿƾͱ����ˣ�ѡ��C��ȷ����ѡC��(4)�������Ҵ���Ӧ�Ļ�ѧ����ʽΪ��
2CH3CHO��2H2O��(3)A����CH2OHԭ���ſɱ�����KMnO4��Һ������ѡ��A����B���Ҵ�Ҳ��ֱ��������CH3COOH��ѡ��B����C���ƾ���ijЩ����ʹ�Ҵ�����Ϊ���ᣬ���Ǿƾͱ����ˣ�ѡ��C��ȷ����ѡC��(4)�������Ҵ���Ӧ�Ļ�ѧ����ʽΪ��  �����ɵ�ˮΪH
�����ɵ�ˮΪH![]() O����ˮ����Է�������Ϊ20��
O����ˮ����Է�������Ϊ20��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ���(����)
��� | ʵ����� | ʵ��Ŀ�� |
�� | �μ����Ը��������Һ | ȷ�����л�����ȩ |
�� | ��ˮ����������Һ��ֱ�Ӽ�������������ͭ����Һ������ | ȷ�������Ƿ�ˮ�� |
�� | ����̼��������Һ | ȷ���Ҵ��л������� |
�� | �ӵ��� | ȷ��ʳ���к��е���� |
A. �٢� B. �ڢ� C. �٢� D. �ڢ�