��Ŀ����
 �£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���1����֪��25�棬101kPaʱ��16.0g N2H4����������ȫȼ�����ɵ������ų�����312kJ��N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol
N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol
����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ����-����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ�ǣ�N2H4+4OH--4e-=4H2O+N2
N2H4+4OH--4e-=4H2O+N2
����ع���һ��ʱ��������Һ��pH����С
��С
�����������С���������䡱������2����ͼ��һ���绯ѧװ��ʾ��ͼ������--����ȼ�ϵ������װ�õĵ�Դ�����A�Dz��缫��B��ʯī�缫��C��500mL�����ı����Ȼ�����Һ��������������1.12L����ʱ����Һ��pHΪ
13
13
������-����ȼ�ϵ�����������ĵĿ���1.4
1.4
L����������ڱ�״���²ⶨ����������������������Ϊ20%����3����������ˮ�������백���Ƶ�����õ��뷽��ʽ��ʾ�µ�ˮ��Һ�Լ��Ե�ԭ��
N2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-
N2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-
����4�������½�0.2mol/L HCl��Һ��0.2mol/L N2H4?H2O��Һ�������ϣ����Ի�Ϻ���Һ����ı仯��������û����Һ��pH=6��������Һ����ˮ�������c��H+��
����
����
0.1mol/L HCl��Һ����ˮ�������c��H+��������ڡ�����С�ڡ������ڡ�������5����֪������ͬ������N2H4?H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ������£�����0.2mol/L N2H4?H2O��Һ��0.1mol/L HCl��Һ�������ϣ�����Һ��N2H+5��Cl-��OH-��H+����Ũ���ɴ�С��˳��Ϊ
c��N2H5+����c��Cl-����c��OH-����c��H+��
c��N2H5+����c��Cl-����c��OH-����c��H+��
����������1�������ºͷ�Ӧ�ȵĹ�ϵ�������ȼ���ȣ���д������Ӧ���Ȼ�ѧ����ʽ��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ��������Һ������������Ũ�ȵı仯�ж���ҺpHֵ�ı仯��
��2�����ݵ�ط�Ӧʽȷ�������������������������������������Ƶ�Ũ�ȣ���ˮ�����ӻ���������������Ũ�ȣ��Ӷ�ȷ����Һ��pHֵ��
��3����������ˮ�������백���Ƶ�������������OH-�������ӣ�
��4�������£������Һ��pH=6����Һ�����ԣ������������������Ӵ���ˮ��������������ӣ�
��5����0.2mol/L N2H4?H2O��Һ��0.1mol/L HCl��Һ�������ϣ��õ�������N2H4?H2O��N2H5C1������ͬ������N2H4?H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ�����Һ�Լ��ԣ���c��OH-����c��H+����
��2�����ݵ�ط�Ӧʽȷ�������������������������������������Ƶ�Ũ�ȣ���ˮ�����ӻ���������������Ũ�ȣ��Ӷ�ȷ����Һ��pHֵ��
��3����������ˮ�������백���Ƶ�������������OH-�������ӣ�
��4�������£������Һ��pH=6����Һ�����ԣ������������������Ӵ���ˮ��������������ӣ�
��5����0.2mol/L N2H4?H2O��Һ��0.1mol/L HCl��Һ�������ϣ��õ�������N2H4?H2O��N2H5C1������ͬ������N2H4?H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ�����Һ�Լ��ԣ���c��OH-����c��H+����
����⣺��1��16.0g�µ����ʵ���=
=0.5mol��0.5molN2H4����������ȫȼ�����ɵ����ų�����312kJ����1mol������������ȫȼ�շ��ȵ�����Ϊ624KJ���������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��ȼ�ϵ���и�����Ͷ�ŵ���ȼ���£���������ʧ���Ӻ����������ӷ�Ӧ����ˮ�͵������缫��ӦʽΪ��N2H4+4OH--4e-=4H2O+N2����Ӧ����������ˮ������Һ������������Ũ�ȼ�С��������Һ��pHֵ��С��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��N2H4+4OH--4e-=4H2O+N2����С��
��2�����ʳ��ˮ�ķ���ʽΪ��2NaCl+2H2O
2NaOH+Cl2��+H2�������ݷ���ʽ֪�������������ɵ��������ʵ�����ȣ������Ҳ��ȣ��������������������0.56L������0.56L����ͬʱ�����������Ƶ����ʵ���=
��2=0.05mol���������Ƶ����ʵ���Ũ��=
=0.1mol/L��������Ũ��Ϊ10-13 mol/L��������Һ��pH=13��
���ݵ�ʧ��������ȣ�����0.56L����ת�Ƶĵ����������������ת�Ƶĵ�������ȣ�����������ΪXL��
0.56L��2=0.2XL��4��X=1.4��
�ʴ�Ϊ��13��1.4��
��3����������ˮ�������백���Ƶ�������������OH-�������ӣ�
���뷽��ʽΪN2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-��
�ʴ�Ϊ��N2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-��
��4�������£������Һ��pH=6����Һ�����ԣ������������������Ӵ���ˮ��������������ӣ���ˮ�������
c��H+��Ϊ10-8mol/L��0.1mol/LHCl��Һ����ˮ�������c��H+��Ϊ
=10-13mol/L��
�ʴ�Ϊ�����ڣ�
��5����0.2mol/L N2H4?H2O��Һ��0.1mol/L HCl��Һ�������ϣ��õ�������N2H4?H2O��N2H5C1������ͬ������N2H4?H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ���c��N2H+5����c��Cl-��������Һ�Լ��ԣ���c��OH-����c��H+�������롢ˮ��ij̶ȶ���������c��N2H5+����c��Cl-����c��OH-����c��H+����
�ʴ�Ϊ��c��N2H5+����c��Cl-����c��OH-����c��H+����
| 16g |
| 32g/mol |
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��N2H4+4OH--4e-=4H2O+N2����С��
��2�����ʳ��ˮ�ķ���ʽΪ��2NaCl+2H2O
| ||
| 0.56L |
| 22.4L/mol |
| 0.05mol |
| 0.5L |
���ݵ�ʧ��������ȣ�����0.56L����ת�Ƶĵ����������������ת�Ƶĵ�������ȣ�����������ΪXL��
0.56L��2=0.2XL��4��X=1.4��
�ʴ�Ϊ��13��1.4��
��3����������ˮ�������백���Ƶ�������������OH-�������ӣ�
���뷽��ʽΪN2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-��
�ʴ�Ϊ��N2H4+H2O=N2H5++OH-��N2H5++H2O=N2H62++OH-��
��4�������£������Һ��pH=6����Һ�����ԣ������������������Ӵ���ˮ��������������ӣ���ˮ�������
c��H+��Ϊ10-8mol/L��0.1mol/LHCl��Һ����ˮ�������c��H+��Ϊ
| 10-14 |
| 0.1 |
�ʴ�Ϊ�����ڣ�
��5����0.2mol/L N2H4?H2O��Һ��0.1mol/L HCl��Һ�������ϣ��õ�������N2H4?H2O��N2H5C1������ͬ������N2H4?H2O�ĵ���̶ȴ���N2H5C1��ˮ��̶ȣ���c��N2H+5����c��Cl-��������Һ�Լ��ԣ���c��OH-����c��H+�������롢ˮ��ij̶ȶ���������c��N2H5+����c��Cl-����c��OH-����c��H+����
�ʴ�Ϊ��c��N2H5+����c��Cl-����c��OH-����c��H+����
����������Ϊ�ۺ���ϰ�⣬�����˹���ɡ��Ȼ�ѧ��Ӧ����ʽ���绯ѧ�����롢ˮ�⡢����Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬�ѶȽϴ�ע�ظ߿��ȵ�Ŀ��鼰֪ʶǨ��������ѵ����

��ϰ��ϵ�д�
�����Ŀ
 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ� �£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ�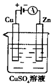 �£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ�������
�£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ�������