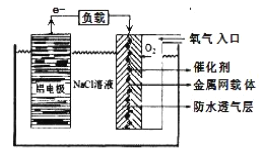
��Ŀ����
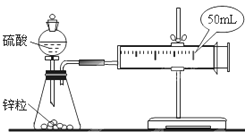
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У����������Һ�¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ����������¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
(1)����NaOH��Һ����ȷ������_________
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
(2)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__________
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò��������� C����������ձ� D���������¶ȼ��ϵĻ��β�������������س鶯
(3)ʵ���������±���
�¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ٸ��ݱ������ݼ�������¶Ȳ��ƽ��ֵΪ______����
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��_______( ȡС�����һλ)��
������ʵ����ֵ������к���Ϊ57.3 kJ/mol��ƫ�����ƫ���ԭ�������____��
a��ʵ��װ�ñ��¡�����Ч���� b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� c����ȡNaOH��Һ�����ʱ���Ӷ��� d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
���𰸡�C D 3.4 -56.8kJ/mol abcd
��������
��ʵ���Ŀ���Dzⶨ�к��ȣ��к�����ָ��ǿ���ǿ���ϡ��Һ��ȫ��Ӧ����1molˮ�ų�����������ʵ�������Ȳⶨ��ͼӦǰ���¶ȣ�Ȼ��ⶨ��Ӧ��ֹ�¶ȣ�Ȼ�����ñ����ݽ��¶�ת��Ϊ����������к��ȡ���ʵ����Ϊ��֤��ͼ���ȫ��Ӧ��NaOH������ʵ��Ĺؼ���Ҫ���¡�
(1)Ϊ�˼���������ɢʧ��ʵ������е���NaOH��Һʱ������һ��Ѹ�ٵĵ��룬����ѡC��
(2)�¶ȼ��Dz����¶ȵģ�����ʹ���¶ȼƽ��裻Ҳ������������ձ���������ܵ���Һ�彦��������ɢʧ��Ӱ��ⶨ����������ܴ�ӲֽƬ�ò��������裬�����������ɢʧ��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ���������ѡD��
(3)���Ĵε��²�ֱ�Ϊ3.4����5.1��3.3����3.5������2������ƫ��ϴ���ȥ������ƽ���²�Ϊ![]() =3.4����
=3.4����
��50mL0.25mol/L������50mL0.55mol/LNaOH��Һ�����кͷ�Ӧ��NaOH��������������ˮ�����ʵ���Ϊ0.05L��0.25mol/L��2=0.025mol����Һ������Ϊ100ml��1g/cm3=100g���¶ȱ仯��ֵ��T=3.4����������0.025molˮ�ų�������ΪQ=mc��T=100g��4.18J/(g��)��3.4��=1421.2J����1.4212kJ������ʵ���õ��к��ȡ�H=-![]() =-56.8kJ/mol��
=-56.8kJ/mol��
��ʵ�����ľ���ֵС���к��ȵľ���ֵ��
a��ʵ��װ�ñ��¡�����Ч�������ɢʧ�ϴ������к��ȵľ���ֵƫС����a���ϣ�
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��¶ȼ���մ�е�NaOH�����ᷴӦ�ų�������ʹ�òⶨ�ij�ʼ�¶�ƫ�ߣ������������ɢʧ���ⶨ���²�ƫС���к��ȵľ���ֵƫС����b���ϡ�
c����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������ƫ�������������������ɵ�ˮ�����ʵ������䣬�ų������������䣬�������NaOH��Һ���ƫ��ʹ���Һ������ƫ�����õ��²��ƫС���к��ȵľ���ֵƫС����c���ϣ�
d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�ϴ������к��ȵľ���ֵƫС����d���ϣ�
�ʴ�Ϊ��abcd��

����Ŀ��ijͬѧ����SO2������ʵ�顣�ڵ�ΰ�a��b��c��d���ֱ���в�ͬ���Լ�������Na2SO3�����ϵμ�����ŨH2SO4����������ΰ��ϸ���������һ��ʱ���۲쵽��ʵ�����������ʾ������˵����ȷ���ǣ� ��
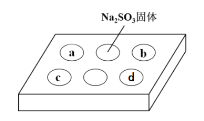
��� | �Լ� | ʵ������ |
a | Ʒ����Һ | ��ɫ��ȥ |
b | ����KMnO4��Һ | ��ɫ��ȥ |
c | NaOH��Һ����2�η�̪�� | ��ɫ��ȥ |
d | H2S��Һ | ��ɫ���� |
A.��Ũ������Na2SO3���巴Ӧ�У�Ũ������ֵ�ǿ������
B.a��b������SO2����Ư����
C.c��ֻ���ܷ�����Ӧ��SO2+2OH-=SO32-+H2O
D.d���SO2����������
����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)+H2(g)CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ�����
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
(1)�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=___����֪��K1000����K800������÷�Ӧ��__��Ӧ��(��������������������)��
(2)��֪��800��ʱ���÷�Ӧ��ƽ�ⳣ��K1=0.9������¶��·�ӦCO(g)��H2O(g)![]() CO2(g)��H2(g)��ƽ�ⳣ��K2=___��
CO2(g)��H2(g)��ƽ�ⳣ��K2=___��
(3)���жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������__��
A��������ѹǿ����
B�����������c(CO)����
C��v��(H2)=v��(H2O)
D��c(CO2)=c(CO)
(4)ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c(CO2)c(H2)=c(CO)c(H2O)�����жϴ�ʱ���¶�Ϊ__�档
����Ŀ����15�֣���̼���ʵļ�ֵ��ת���������ڡ���̼���Ϳɳ����Է�չ��������Ҫ���о���ֵ����ش��������⣺
��1����֪CO�����л�ѧ��ΪC��O����صĻ�ѧ�������������£�
��ѧ�� | H��O | C��O | C=O | H��H |
E/(kJ��mol1) | 463 | 1075 | 803 | 436 |
CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H=___________kJ��mol1���������������COƽ��ת���ʵĴ�ʩ��_______________�����ţ���
CO2(g)��H2(g) ��H=___________kJ��mol1���������������COƽ��ת���ʵĴ�ʩ��_______________�����ţ���
a������ѹǿ b�������¶�
c�����ԭ������H2O�ı��� d��ʹ�ø�Ч����
��2���ö��Ե缫���KHCO3��Һ���ɽ������е�CO2ת��Ϊ�����(HCOO)��Ȼ���һ�������Ƶ���Ҫ�л�����ԭ�ϼ��ᡣCO2������Ӧ�ĵ缫��ӦʽΪ________________������������ת��1 mol���ӣ���������������������״����Ϊ_________L��
��3���ұ���������ȡ����ϩ�ķ�ӦΪ��![]() (g)��CO2(g)
(g)��CO2(g)![]()
![]() (g)��CO(g)��H2O(g)���䷴Ӧ�������£�
(g)��CO(g)��H2O(g)���䷴Ӧ�������£�
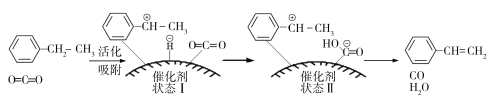
����ԭ�ϵ�״̬��____________��������ų��������ա�����
��һ���¶��£�������ܱ������г���2 mol�ұ���2 mol CO2����ʼѹǿΪp0��ƽ��ʱ���������������ʵ���Ϊ5 mol���ұ���ת����Ϊ_______����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=_______��[�����ѹ(p��)=������ѹ(p��)�������������]
���ұ�ƽ��ת������p(CO2)�Ĺ�ϵ����ͼ��ʾ��������ұ�ƽ��ת��������p(CO2)�仯���仯��ԭ��________________________________��