��Ŀ����
����Ŀ��ijͬѧ����SO2������ʵ�顣�ڵ�ΰ�a��b��c��d���ֱ���в�ͬ���Լ�������Na2SO3�����ϵμ�����ŨH2SO4����������ΰ��ϸ���������һ��ʱ���۲쵽��ʵ�����������ʾ������˵����ȷ���ǣ� ��
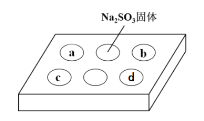
��� | �Լ� | ʵ������ |
a | Ʒ����Һ | ��ɫ��ȥ |
b | ����KMnO4��Һ | ��ɫ��ȥ |
c | NaOH��Һ����2�η�̪�� | ��ɫ��ȥ |
d | H2S��Һ | ��ɫ���� |
A.��Ũ������Na2SO3���巴Ӧ�У�Ũ������ֵ�ǿ������
B.a��b������SO2����Ư����
C.c��ֻ���ܷ�����Ӧ��SO2+2OH-=SO32-+H2O
D.d���SO2����������
���𰸡�D
��������
a.Ʒ����Һ��ɫ��˵��SO2����Ư���ԣ�b.���Ը��������Һ��ɫ��˵��SO2���л�ԭ�ԣ�c. ���з�̪��NaOH��Һ��ɫ˵��SO2Ϊ�������壻d. SO2�������ᷴӦ���ɻ�ɫ��S���ʣ�˵������������ԡ�
A. Ũ������Na2SO3���巴Ӧ��ȡSO2Ϊ���ֽⷴӦ�����漰������ԭ��A����
B. SO2����ʹƷ����ɫ��˵�������Ư���ԣ�SO2ʹ���Ը��������Һ��ɫ���������仹ԭ�ԣ�B����
C. c�п��ܷ���SO2+2OH-=SO32-+H2O��Ҳ���ܷ���SO2+OH-=HSO3-��C����
D. SO2�������ᷴӦ���ɻ�ɫ��S���ʣ�˵������������ԣ�D��ȷ��
�ʴ�ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У����������Һ�¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ����������¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
(1)����NaOH��Һ����ȷ������_________
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
(2)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__________
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò��������� C����������ձ� D���������¶ȼ��ϵĻ��β�������������س鶯
(3)ʵ���������±���
�¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ٸ��ݱ������ݼ�������¶Ȳ��ƽ��ֵΪ______����
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��_______( ȡС�����һλ)��
������ʵ����ֵ������к���Ϊ57.3 kJ/mol��ƫ�����ƫ���ԭ�������____��
a��ʵ��װ�ñ��¡�����Ч���� b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� c����ȡNaOH��Һ�����ʱ���Ӷ��� d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���