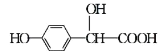
��Ŀ����
����Ŀ����ͼ��һ����ѧ���̵�ʾ��ͼ��
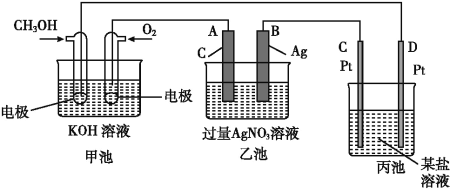
(1)C(Pt)�缫��������____��
(2)д��ͨ��O2�ĵ缫�ϵĵ缫��Ӧʽ:_______________��
(3)д��ͨ��CH3OH�ĵ缫�ϵĵ缫��Ӧʽ:_________��
(4)�������ǵ�ⱥ��ʳ��ˮ��Һ,��____(��������������������)���������̪��Һ��졣
(5)�ҳ��з�Ӧ�Ļ�ѧ����ʽΪ____��
(6)���ҳ���B(Ag)������������5.40 gʱ,�׳�������������O2____mL(��״����);�������б���ʳ��ˮ��Һ�����Ϊ500 mL,����,��Һ��pH=_____��(25 ��,������ǰ����Һ������ޱ仯)��
���𰸡����� O2+4e-+2H2O=4OH- CH3OH+8OH--6e-=CO32-+6H2O ���� 4AgNO3+2H2O![]() 4Ag+O2��+4HNO3 280 13
4Ag+O2��+4HNO3 280 13
��������
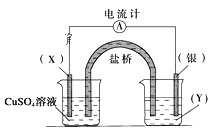
ȼ�ϵ���У�ͨ��ȼ�ϼ״��ĵ缫Ϊ�������缫��ӦΪCH3OH+8OH--6e-=CO32-+6H2O��ͨ���������ĵ缫Ϊ������������ӦΪO2+4e-+2H2O=4OH-���ҳء������ǵ��أ����Դ���������������������Դ��������������������A��������B���������ҳ�������A���������ŵ��������������B�������ӷŵ��������������C��������D���������������Һ���Ȼ�����Һ���������������Ϸŵ磬�������������Ϸŵ磬�ݴ˷������
��������������֪�׳�Ϊԭ��أ��ҡ�����Ϊ����ʡ��ڼ׳ص�ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫Ϊ������ͨ�������ĵ缫Ϊ���������Դ���������������������Դ��������������������A��������B��������������C��������D��������
(1)��������������֪C(Pt)���ӵ�Դ��������Ϊ������
(2)ͨ��O2�ĵ缫��������������O2��õ��ӣ�����Һ�е�H2O����γ�OH-���缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
(3)ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫Ϊ�������缫��ӦΪ��CH3OH+8OH--6e-=CO32-+6H2O��
(4)�������ǵ�ⱥ��ʳ��ˮ��Һ���ڱ�����C��������D���������������Һ���Ȼ�����Һ��Cl-������ʧ���Ӳ���Cl2���缫��ӦΪ��2Cl--2e-=Cl2����H+�������õ����Ӳ���H2���缫��ӦΪ��2H++2e-=H2����H+�ŵ磬ˮ�ĵ���ƽ�ⱻ�ƻ���ʹ������Һ��OH-��Ũ������������Һ�ʼ��ԣ������̪��Һ��죬�������������Һ��Ϊ��ɫ��
(5)�ҳ���A��������B��������������ˮ�������������ʧ���Ӳ������������������ӵõ��Ӳ��������������ڷ��������ͣ���ⷴӦ�Ļ�ѧ����ʽΪ��4AgNO3+2H2O![]() 4Ag+O2��+4HNO3��
4Ag+O2��+4HNO3��
(6)���ҳ���B(Ag)������������5.40gʱ������Ag++e-=Ag��ת�Ƶ��ӵ����ʵ���n(e-)= 5.40g��108g/mol=0.05mol������ͬһ�պϻ�·�е���ת����Ŀ��ȣ����O2+4e-+2H2O=4OH-����֪�ڼ׳�������������O2�������V(O2)=![]() ��22.4L/mol=0.28L=280mL����Ӧ���������������ʵ���Ϊ0.05mol��c(OH-)=
��22.4L/mol=0.28L=280mL����Ӧ���������������ʵ���Ϊ0.05mol��c(OH-)=![]() =0.1mol/L��������ҺpH=13��
=0.1mol/L��������ҺpH=13��

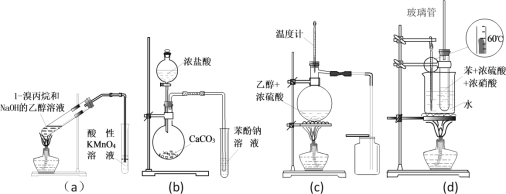
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
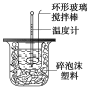
������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У����������Һ�¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ����������¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
(1)����NaOH��Һ����ȷ������_________
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
(2)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__________
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò��������� C����������ձ� D���������¶ȼ��ϵĻ��β�������������س鶯
(3)ʵ���������±���
�¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ٸ��ݱ������ݼ�������¶Ȳ��ƽ��ֵΪ______����
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��_______( ȡС�����һλ)��
������ʵ����ֵ������к���Ϊ57.3 kJ/mol��ƫ�����ƫ���ԭ�������____��
a��ʵ��װ�ñ��¡�����Ч���� b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� c����ȡNaOH��Һ�����ʱ���Ӷ��� d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���