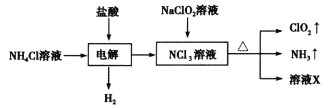
��Ŀ����
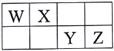
����Ŀ��X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������X��Zͬ���壻���γ����ӻ�����ZX��Y��Mͬ���壻���γ�MY2��MY3���ַ��ӡ�
��ش��������⣺
��1��Y��Ԫ�����ڱ��е�λ��Ϊ___��
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ����___(д��ѧʽ)���ǽ�����̬�⻯�ﻹԭ����ǿ����___(д��ѧʽ)��
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������___(д�������������ʵĻ�ѧʽ)��
��4��X2M��ȼ������H=-a kJ��mol-1��д��X2Mȼ�շ�Ӧ���Ȼ�ѧ����ʽ��_____��
��5��ZX�ĵ���ʽΪ________��ZX��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ_______��
���𰸡��ڶ�������A�� HClO4 H2S ClO2��Cl2��O3 2H2S(g)+3O2(g)=2H2O(l)+2SO2(g)��Hh=��2akJ/mol ![]() NaH+H2O=NaOH+H2��
NaH+H2O=NaOH+H2��
��������
������Ҫ����Ԫ�����ڱ���Ԫ�������ɡ����������֪X��Y��Z��M��G�ֱ����⡢�����ơ����ȡ�
��1��Y��Ԫ�����ڱ��е�λ��Ϊ�ڶ����ڡ���A�塣
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ���Ƿǽ�������ǿ����Ԫ���γɵ�HClO4���ǽ�����̬�⻯�ﻹԭ����ǿ���Ƿǽ�������������Ԫ���γɵ�H2S��
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������ClO2��Cl2��O3��
��4��X2M��ȼ������H=-a kJ��mol-1��X2Mȼ�շ�Ӧ���Ȼ�ѧ����ʽ��2H2S(g)+3O2(g)=2H2O(l)+2SO2(g)��H=��2akJ/mol��
��5��ZX�������ӻ���������ʽΪ![]() ��ZX��ˮ��Ӧ��NaH��H(��-1��)��H2O��H(��+1��)����������ԭ��Ӧ��������������Ӧ�Ļ�ѧ����ʽΪNaH+H2O=NaOH+H2����
��ZX��ˮ��Ӧ��NaH��H(��-1��)��H2O��H(��+1��)����������ԭ��Ӧ��������������Ӧ�Ļ�ѧ����ʽΪNaH+H2O=NaOH+H2����