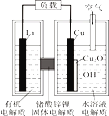
��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣�������ŷֱ����ijһԪ�ء���ش��������⣮
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
��1�������뵼����ϵ�Ԫ���� ______����Ԫ�ط��ţ�����̬�⻯����������������ˮ�����ֱ�ӻ�������һ���ε�Ԫ���� _____�������ƣ���
��2���������������Ӱ뾶��С����___________�������ӷ��ţ���
��3���� ~ �������������ˮ�����У�������ǿ���� __________���ѧʽ����������ǿ����________���ѧʽ�������������������ˮ���ﷴӦ�����ӷ���ʽΪ__________��
��4���ࡢ����γ�A2B2�͵Ļ���������ж��߾�����8�����ȶ��ṹ���������ʽΪ_________________��
���𰸡�Si �� Al3+ HClO4 NaOH Al(OH)3 +OH��= AlO2��+ 2H20 ![]()
��������
ѧ��Ԫ�����ڱ��������Ҫ��һ��Ԫ�����ڱ��Ŀ�ܽṹͼ��Ҫ��Ϥ��������ÿ��Ԫ�صľ���λ�á�
����ʱҪ�������������ÿ��Ԫ�ص������ڱ��еľ���λ�á�����C������N������O������F������Na������Al������Si������S������Cl������Ar��
(1)�����뵼����ϵ�Ԫ�ش��ڽ�����ǽ����ֽ��߸�����Ԫ�أ����ǹ裬Ԫ�ط���ΪSi����̬�⻯����������������ˮ�����ֱ�ӻ�������һ���ε�Ԫ���ǵ���NH3+HNO3=NH4NO3��
(2)�ϱ��е�������Ԫ�����γɼ����ӵ���Na+��Al3+��S2-��Cl-��Na+��Al3+���Ӳ�ṹ��ͬ��S2-��Cl-���Ӳ�ṹ��ͬ�����Ӳ�ṹ��ͬ�����ӣ�ԭ��������İ뾶С������r(Na+)>r(Al3+)��r(S2-)>r(Cl-)��Cl-��Na+��һ���Ӳ㣬r(Cl-)>r(Na+)���������Ӱ뾶��С����Al3+��
(3)�ǽ�����Խǿ��Ԫ�ص�����������ˮ���������Խǿ��������Խǿ����������ˮ����ļ���Խǿ�������ϱ��зǽ�����ǿ���Ƿ�������Ԫ��û�����ۣ������γ�ͨ�������ϵĺ����ᣬ����������ǿ����HClO4(������)���ϱ��н�������ǿ��Ԫ�����ƣ����Լ�����ǿ����NaOH��������������ˮ������Al(OH)3��NaOH��Al(OH)3��Ӧ�����ӷ���ʽ��OH-+Al(OH)3=AlO2-+2H2O��
(4)S��Cl��Ԫ�ؿ��γ�S2Cl2�Ļ�ѧ�Sԭ���������6�����ӣ��ﵽ8���ӽṹ��Ҫ�γ�2�Թ��õ��Ӷԣ�Clԭ���������7�����ӣ��ﵽ8���ӽṹ���γ�1�Թ��õ��Ӷԣ����ֻ������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Ȼ��ÿ��S�ٷֱ���1��Cl�γ�1�Թ��õ��Ӷԣ����Ե���ʽ��![]() ��
��