��Ŀ����
11��������ʵ����ơ�ij�о���ѧϰС��������ϻ�þ���M���Ʊ�ԭ�������ǽ�������̽�������Ʊ����塿
��CrCl2•4H2O���������⡢Һ�����Ȼ�粒���Ϊԭ�ϣ��ڻ���̿���£��ϳ��˾���M��
��1����Һ�з��������M�IJ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӻ�������ñ�ˮ�ͱ���ʳ��ˮ�Ļ����ϴ�Ӿ���M����Ŀ���ǽ��;����ܽ�ȣ��Ʊ���������Ҫ���ȣ����ǣ��¶ȹ�����ɵĺ���Ǽӿ�˫��ˮ�ֽ��Һ���ӷ���
���ⶨ��ɡ�
Ϊ�˲ⶨM������ɣ������������ʵ�飮װ����ͼ��ʾ�����������̶�����ʡ�ԣ���
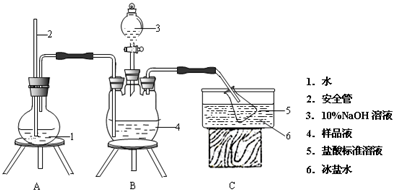
Ϊȷ������ɣ���������ʵ�飺
�ٰ��IJⶨ����ȷ��M���壬������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������20% NaOH��Һ��ͨ��ˮ����������Ʒ��Һ�еİ�ȫ����������һ������������Һ���գ�����������ȡ�½���ƿ����һ��Ũ�ȵ�NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����һ�������NaOH��Һ��
���ȵIJⶨ��ȷ��ȡa g��ƷM��������ˮ�����100mL��Һ����ȡ25.00mL���Ƶ���Һ��c mol•L-1AgNO3����Һ�ζ����μ�3��0.01mol•L-1K2CrO4��Һ����ָʾ������������ש��ɫ����������ʧΪ�յ㣨Ag2CrO4Ϊש��ɫ��������AgNO3��ҺΪb mL��
��2����ȫ�ܵ�������ƽ����ѹ��
��3�����������Ʊ���Һ�ζ��������Ȼ��⣬���ֲ��������Ǽ��ζ����Ƿ�©Һ��������ˮϴ�ӡ��ñ�NaOH��Һ��ϴ���ŵζ��ܼ�������ݡ����ڼ�ʽ�ζ�����Һ����0�̶Ȼ�0�̶����¡��ζ�����������¼���������ݣ����в����������ʹ�ⶨ��Ʒ��NH3����������ƫ�ߵ���C������ţ���
A��װ�������Բ��� B���÷�̪��ָʾ��
C���ζ��յ�ʱ���Ӷ��� D���ζ�ʱNaOH��Һ�⽦
��4����֪�����������ȶ��ԲKsp��Ag2CrO4��=1.12��10-12��Ksp��AgCl��=1.8��10-10
ѡ����ɫ�ζ���ʢװ��Ũ�ȵ���������Һ���ζ��յ�ʱ������Һ��
c��CrO42-��Ϊ2.8��10-3mol•L-1����c��Ag+��=2.0��10-5mol•L-1��
��5����������ʵ�����ݣ��г���ƷM����Ԫ��������������ʽ$\frac{cmol/L��\frac{b}{1000}L��35.5g/mol}{ag��\frac{25.00mL}{100mL}}��100%$������μ�K2CrO4��Һ���࣬��ý����ƫ�ͣ��ƫ�ߡ�ƫ�ͻ���Ӱ�죩��
��6�����ⶨ������M�и��������ȵ�����֮��Ϊ104��136��213��д���Ʊ�M����Ļ�ѧ����ʽ2CrCl2+H2O2+6NH3+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$2Cr��NH3��4Cl3+2H2O��
���� ���Ʊ����塿
��1��������ʳ��ˮ�¶ȵͣ������ܽ��С���ñ�����ʳ��ˮϴ�Ӿ��壬��С�����ܽ⣬˫��ˮ�����ֽ⣬Һ���ӷ���
���ⶨ��ɡ�
��2����ȫ�ܵ�������ƽ����ѹ��ʹ��ƿ����ѹǿƽ�⣻
��3�����������Ʊ���Һ�ζ��������Ȼ��⣬��ʽ�ζ������ñ�Һ��ϴ���ζ���0�̶��ڵζ��ܵ��Ϸ���A��װ�������Բ��ã����ְ����ݳ��� B��ǿ��ǿ��ζ����÷�̪��ָʾ����Ӱ�죻C���ζ��յ�ʱ���Ӷ���������С��ʵ������� D���ζ�ʱNaOH��Һ�⽦����ҺNaOH��Һ�������ƫ���²��ʣ���������ƫ��
��4���������ֽ⣬����Ũ���ᣬ�������ڹ��յ��������ֽ⣬����������������������������Ksp��Ag2CrO4��=c2��Ag+��•c��CrO42-�����㣻
��5������Ag++Cl-=AgCl��s������ƷM����Ԫ���������������ӵ��������ݴ˼�����ƷM����Ԫ���������������ݸ��������Ȼ������ܶȻ�֪�����������ܽ�ȱ��Ȼ����Դ�һЩ������μӸ������Һ���࣬����ǰ���ɸ���������������û����ȫת�����Ȼ�����������ǰָʾ�յ㣬�����Ԫ����������ƫ�ͣ�
��6������M�и��������ȵ�����֮��Ϊ104��136��213�����������Ԫ�ص����ʵ���֮�ȣ����M�Ļ�ѧʽ�����ݻ�ѧʽ��д����ʽ��
��� �⣺���Ʊ����塿
��1��������������˵õ����壬�ñ�����ʳ��ˮϴ�Ӿ��壬Ŀ���Ǽ��پ���M�ܽ�����˫��ˮ�����ֽ�2H2O2$\frac{\underline{\;\;��\;\;}}{\;}$2H2O+O2����Һ���ӷ��������¶ȹ��ߣ����·�Ӧ����ʧ��
�ʴ�Ϊ�����;����ܽ�ȣ��ӿ�˫��ˮ�ֽ��Һ���ӷ���
���ⶨ��ɡ�
��2��Aװ�������ṩˮ��������Ҫ���ȣ�������ƽ����ѹ���ã��ʴ�Ϊ��ƽ����ѹ��
��3���ζ��ܾ���©֮���ñ�����������Һ��ϴ���ζ���0�̶����Ϸ��������Һ����0�̶ȼ�0�̶����²ſɶ�����
A��װ��©�������ְ�����ɢ�ˣ��ⶨ��NH3��������ƫ�ͣ���A����
B��ǿ��ζ�ǿ�����ѡ����������̪��ָʾ�������ᵼ������B����
C���ζ��յ�ʱ���Ӷ���������С��ʵ��������ⶨ��������������Һ���ƫС��NH3��������ƫ�ߣ���C��ȷ��
D���ζ�Һ�⽦�����²��ʣ���������ƫ��NH3��������ƫС����D����
�ʴ�Ϊ���ñ�NaOH��Һ��ϴ�����ڼ�ʽ�ζ�����Һ����0�̶Ȼ�0�̶����£�C��
��4���������ܲ��ȶ����ڹ��յ��������ֽ⣺2AgNO3$\frac{\underline{\;����\;}}{\;}$2NO2��+O2��+2Ag��Ӧѡ����ɫ�ζ���ʢװ��������Һ��Ksp��Ag2CrO4��=c2��Ag+��•c��CrO42-����c��Ag+��=$\sqrt{\frac{{K}_{sp}��A{g}_{2}Cr{O}_{4}��}{C��Cr{{O}_{4}}^{2-}��}}$=$\sqrt{\frac{1.12��1{0}^{-12}}{2.8��1{0}^{-3}}}$mol•L-1=2.0��10-5mol•L-1��
�ʴ�Ϊ��2.0��10-5mol•L-1��
��5��Ag++Cl-=AgCl��s��������Cl��=$\frac{cmol/L��\frac{b}{1000}L��35.5g/mol}{ag��\frac{25.00mL}{100mL}}��100%$��������Ksp��Ag2CrO4��=1.12��10-12���Ȼ���Ksp��AgCl��=1.8��10-10�����������ܽ�ȱ��Ȼ����Դ�һЩ������μӸ������Һ���࣬����ǰ���ɸ���������������û����ȫת�����Ȼ�����������ǰָʾ�յ㣬�����Ԫ����������ƫ�ͣ�
�ʴ�Ϊ��$\frac{cmol/L��\frac{b}{1000}L��35.5g/mol}{ag��\frac{25.00mL}{100mL}}��100%$��ƫ�ͣ�
��6��n��Cr����n��NH3����n��Cl��=$\frac{104}{52}$��$\frac{136}{17}$��$\frac{213}{35.5}$=1��4��3��M�Ļ�ѧʽΪCr��NH3��4Cl3��M�и�Ԫ�ػ��ϼ�Ϊ+3����ѧ����ʽΪ2CrCl2+H2O2+6NH3+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$ 2Cr��NH3��4Cl3+2H2O��
�ʴ�Ϊ��2CrCl2+H2O2+6NH3+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$2Cr��NH3��4Cl3+2H2O��
���� ���⿼���˶���ʵ�鷽������ƣ����������ʵ��Ʊ�ʵ���������ɵIJⶨ�������ܽ�ƽ��ȣ���ȷÿһ���̷����ķ�Ӧ�ǽⱾ��ؼ��������ڻ���֪ʶ���ۺ�Ӧ�õĿ��飬��Ŀ�Ѷ��еȣ�

| A�� | Na2SiO3 | B�� | BaCl2 | C�� | AgNO3 | D�� | NaAlO2 |
| A�� | 39g | B�� | 59g | C�� | 78g | D�� | 97g |
| A�� | ���³�ѹ�£�17g����-14CH3��������������Ϊ9NA | |
| B�� | 1 L 0.2 mol•L-1��������Һ�к��е�SO42-��Ϊ0.2NA | |
| C�� | 0.1mol N2��������H2��Ӧ��ת�Ƶĵ�����Ϊ0.6NA | |
| D�� | �ö��Ե缫���1L0.1mol•L-1 CuCl2��Һ������0.2NA������ͨ��ʱ��������6.4gͭ |
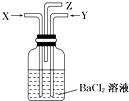 ̼��Ũ���Ṳ�Ȳ���������X��ͭ��Ũ���ᷴӦ����������Y ͬʱͨ��ʢ�������Ȼ�����Һ��ϴ��ƿ�У���ͼ��ʾ���������й�˵����ȷ���ǣ�������
̼��Ũ���Ṳ�Ȳ���������X��ͭ��Ũ���ᷴӦ����������Y ͬʱͨ��ʢ�������Ȼ�����Һ��ϴ��ƿ�У���ͼ��ʾ���������й�˵����ȷ���ǣ�������| A�� | ϴ��ƿ�в����ij�����̼�ᱵ | B�� | ��Z���ܳ�����������������̼ | ||
| C�� | ϴ��ƿ�в����ij��������ᱵ | D�� | ��Z���ܿ��к���ɫ������� |
| A�� | HO-CH2CH2-COOH | B�� | HOOC-COOH | C�� | HO-CH2CH2-OH | D�� | CH2-COOH |
| A�� | ֱ���� | B�� | ���������� | C�� | ����� | D�� | ��֧����ֱ���� |