��Ŀ����
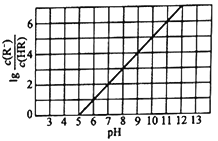
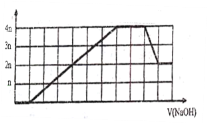
����Ŀ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӡ������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ����
A. ԭ��Һ��һ�����е���������H+��NH4+��Mg2+��Al3+
B. ԭ��Һ��һ������SO42-��Na+
C. ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1
D. ��Ӧ����γɵ���Һ�к��е����ʽ�ΪNa2SO4
���𰸡�C
��������
����ͼ����NaOH��Һʱ��һ��ʼû�г���������������Һ����H+�������������������������ʱ�������μ�NaOH��Һ���������������仯��������Һ����NH4+���ٵμ�NaOH��Һ�����������ܽ⣬������Һ����Al3+�������ܽ����ĵ�NaOH��Һ�����Ϊ1�ݣ�����Al3+����NaOH��ҺΪ3�ݣ���������������NaOH��Һ6�ݣ�����Al(OH)3������һ�ֳ��������ʵ�����ȣ�����һ����Fe3+������Mg2+��
A. ԭ��Һ��һ�����е���������H+��NH4+��Fe3+��Al3+��û��Mg2+����A��ѡ��
B. ԭ��Һ��һ������SO42-��Na+����ȷ������B��ѡ��
C. ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1����Cѡ��
D. ��Ӧ����γɵ���Һ�к��е����ʳ���Na2SO4������NaAlO2����D��ѡ��
��ѡC��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�