��Ŀ����
�״���21����Ӧ����㷺�����ȼ��֮һ��ͨ�����·�Ӧ�����Ʊ��״���
CO(g)+2H2(g)=CH3OH(l) ��H=?
��1����֪��2H2(g)+O2(g)=2H2O(l) ��H=��571.6kJ��mol-1
2CO(g)+O2(g)=2C O2(g) ��H=��566.0kJ��mol-1
O2(g) ��H=��566.0kJ��mol-1
2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(l) ��H=��1453.0kJ��mol-1
���Ʊ��״���Ӧ�ġ�H = kJ��mol-1
��2����װ��Ϊ�ݻ��̶����ܱ���������ͬʱ��θ����ʵ�Ũ�����±���
c(CO) /mol��L-1 | c(H2) /mol��L-1 | c(CH3OH) /mol��L-1 | |
0min | 0.8 | 1.6 | 0 |
2min | 0.6 | y | 0.2 |
4min | 0.3 | 0.6 | 0.5 |
6min | 0.3 | 0.6 | 0.5 |
��Ӧ��2min��4min֮�䣬H2��ƽ����Ӧ����Ϊ________ mol��L��1��min��1��
��Ӧ�ڵ�2minʱ�ı��˷�Ӧ�������ı������������ (����ĸ���)��
A��ʹ�ô��� B�������¶� C������H2��Ũ�� D����СCH3OH(g)��Ũ��
��3�������ݻ��ɱ���ܱ������г���1 mol CO(g)��2 molH2 (g)����CH3OH(g)��H2��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ2 L����ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ �����ﵽƽ��״̬B ʱ�������������V(B)= L��

��4��һ���¶��£����ݻ��̶����ܱ������г���һ������H2��CO����t1ʱ�ﵽƽ�⡣t2ʱ���������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�ﵽ�µ�ƽ��״̬��֮���ٸı�������������ͼ�в�������t2��t4����Ӧ������ʱ��ı仯���ߡ�



 H2��g��+CO2��g����H��0���������������������£�����˵����ȷ���ǣ� ��
H2��g��+CO2��g����H��0���������������������£�����˵����ȷ���ǣ� �� Fe3+��Al3+��Cl����NO
Fe3+��Al3+��Cl����NO B��K����Na����I����SO
B��K����Na����I����SO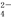
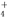 ��NO
��NO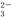 ��SO
��SO l����CO
l����CO ��SO
��SO ��NO3-�е����������ӣ�����Ũ�Ⱦ�Ϊ0.1 mol��L��1��ijͬѧ��������ʵ�飺����˵����ȷ���ǣ� ��
��NO3-�е����������ӣ�����Ũ�Ⱦ�Ϊ0.1 mol��L��1��ijͬѧ��������ʵ�飺����˵����ȷ���ǣ� ��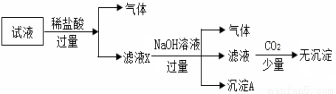
 ��K+��NH4+
��K+��NH4+ ��Al3+��K +��Cl��
��Al3+��K +��Cl�� ��Fe2+��SO
��Fe2+��SO 2NH3(g) ��H =��2a kJ��mol-1
2NH3(g) ��H =��2a kJ��mol-1 2NH3(g) ��H<0
2NH3(g) ��H<0