��Ŀ����
����Ŀ����VA��Ԫ��������������������Ҫ��;���ش���������:
��1������������(�׳ơ����ơ�������ʳƷ������,��ˮ�ֱ��ּ���Ʒ�ʸ������ȡ�
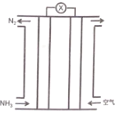
������Ľṹʽ��ͼ��ʾ,����Ҫ�ĵ��뷽��ʽΪ______________��
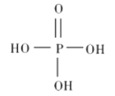
������������������������ȥ������ˮ��IJ���,���������ƵĻ�ѧʽΪ_______________��
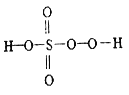
��2���ڼ��������£���������(H2PO2-)�����ڻ�ѧ������д���䷴Ӧ�����ӷ���ʽ______________��(���������뻹ԭ�������ʵ���֮��Ϊ1:4)
��3���ɹ�ҵ����(�������顢����þ��)�Ʊ��ߴ�����(�۵�44��,�е�280��),��Ҫ������������:
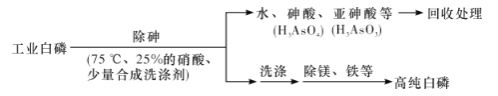
�ٳ��������75 ���½���,�������ԭ����____________(����ĸ����)��
a,ʹ�����ۻ�,������ˮ b.���Ͱ��Ķ���
c.�¶Ȳ��˹���,��ֹ����ֽ� d.�ʵ�����¶�,����Ӧ����
��������������ʱ����ԭΪNO,д����ת��Ϊ������Ļ�ѧ����ʽ:______________________________��
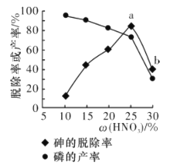
��ij������,��һ���������ᴦ��һ�����Ĺ�ҵ����,����ѳ��ʼ��IJ������������������ı仯��ͼ��ʾ,����ѳ��ʴ�a�㵽b�㽵�͵�ԭ����__________________��
��4��������������Һ�д���ƽ��:Ag+(aq) + 2NH3(aq) ![]() Ag(NH3)2+(aq),K=l.10��107 ;��֪������Ksp(AgCl)=1.45��10-10������淴ӦAgCl(s) +2NH3(aq)
Ag(NH3)2+(aq),K=l.10��107 ;��֪������Ksp(AgCl)=1.45��10-10������淴ӦAgCl(s) +2NH3(aq)![]() Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=_________(����2λ��Ч����)��1L1mol/L��ˮ���������ܽ�AgCl ______mol(����1λ��Ч����)��
Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=_________(����2λ��Ч����)��1L1mol/L��ˮ���������ܽ�AgCl ______mol(����1λ��Ч����)��
���𰸡�![]() Na5P3O10
Na5P3O10 ![]() cd
cd ![]() ����Ũ�ȴ�������ǿ���н϶�����������������ף������ʵ� 1.6��10-3 0.04
����Ũ�ȴ�������ǿ���н϶�����������������ף������ʵ� 1.6��10-3 0.04
��������
��1������������Ԫ���ᣬ�ֲ����룬��Ҫ�ĵ��뷽��ʽΪ![]() ��
��
������������������������ȥ������ˮ��IJ������ԭ���غ��֪��������ķ���ʽ��H5P3O10�������������ǻ�����ԭ�ӱ�������ȡ���������������ƣ����������ƵĻ�ѧʽΪ��Na5P3O10��
��2����ѧ����![]() �����������뻹ԭ�������ʵ���֮��Ϊ1:4������PԪ�ػ��ϼ���+1����Ϊ+5��H2PO2�C������Ϊ
�����������뻹ԭ�������ʵ���֮��Ϊ1:4������PԪ�ػ��ϼ���+1����Ϊ+5��H2PO2�C������Ϊ![]() �����ݵ�ʧ�����غ㣬��Ӧ�����ӷ���ʽ��
�����ݵ�ʧ�����غ㣬��Ӧ�����ӷ���ʽ��![]() ��
��
��3����a.���ײ�����ˮ����a����b.�ۻ����ܸı��䶾�ԣ���b����c.�¶ȹ��ߣ������ֽ⣬��Ҫ���ƺ��ʵ��¶ȣ���c��ȷ��d.����¶ȿ��Լӿ췴Ӧ���ʣ���d��ȷ��ѡcd��
�������������Ϊ�����ᣬ��Ԫ�ػ��ϼ���0����Ϊ+3��NԪ�ػ��ϼ���+5����Ϊ+2�����ݵ�ʧ�����غ㣬��ѧ����Ϊ![]() ��
��
������Ũ�ȴ�������ǿ����a�㵽b�㣬�н϶�����������������ף����������ʵͣ�
��4��Ag+(aq) + 2NH3(aq) ![]() Ag(NH3)2+(aq)��K=l.10��107��
Ag(NH3)2+(aq)��K=l.10��107�� 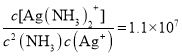 ��Ksp(AgCl)=1.45��10-10����
��Ksp(AgCl)=1.45��10-10����![]() ���淴ӦAgCl(s) +2NH3(aq)
���淴ӦAgCl(s) +2NH3(aq)![]() Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��
Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��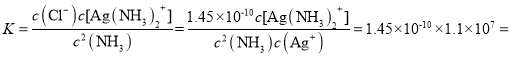 1.6��10-3��
1.6��10-3��
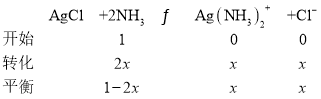
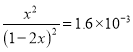 ��x=0.04��1L1mol/L��ˮ���������ܽ�AgCl 0.04mol��
��x=0.04��1L1mol/L��ˮ���������ܽ�AgCl 0.04mol��