��Ŀ����
ˮ��������ԴȪ����ҵ��ѪҺ�����е�������Ҫ�����ú�������ˮ����Ҫ������ˮԴ����Ⱦ��ͨ������ˮ��ֱ�Ӷ������壬Ҳ��ͨ��ʳ�������ũ����Σ��������
��ش��������⣺
(1)��ˮ��100 ��ʱ��pH��6�����¶���1 mol��L��1��NaOH��Һ�У���ˮ�������
c(OH��)��________ mol��L��1��
(2)25 ��ʱ����ˮ�ĵ���ƽ����ϵ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ�ⷽ��ʽΪ__________����ˮ�������c(OH��)��__________ mol��L��1��
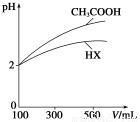
(3)�����Ϊ100 mL��pH��Ϊ2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ���ƽ�ⳣ��________(��������������С��������������)CH3COOH�ĵ���ƽ�ⳣ����������__________________________________��
(4)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ��������������֪��
��ѧʽ | ���볣��(25 ��) |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
��25 ��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ������Һ��pH�ɴ�С��˳��Ϊ____________________________��
����NaCN��Һ��ͨ��������CO2��������Ӧ�Ļ�ѧ����ʽΪ__________________
(5)25 ��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH��6������Һ��c(CH3COO��)��c(Na��)��________ mol��L��1(�ȷֵ)��c(CH3COO��)/c(CH3COOH)��________��
(1)10��12��(2)CO32����H2O HCO3����OH����HCO3����H2O
HCO3����OH����HCO3����H2O H2CO3��OH����10��3��(3)С�ڡ�ϡ����ͬ������һԪ��HX��pH�仯����CH3COOH��С�������Խ���������ƽ�ⳣ����С��(4)��pH(Na2CO3��Һ)��pH(NaCN��Һ)��pH(CH3COONa��Һ)����NaCN��H2O��CO2=HCN��NaHCO3��(5)9.9��10��7��18
H2CO3��OH����10��3��(3)С�ڡ�ϡ����ͬ������һԪ��HX��pH�仯����CH3COOH��С�������Խ���������ƽ�ⳣ����С��(4)��pH(Na2CO3��Һ)��pH(NaCN��Һ)��pH(CH3COONa��Һ)����NaCN��H2O��CO2=HCN��NaHCO3��(5)9.9��10��7��18
��������(1)��ˮ��100 ��ʱ��pH��6����c(H��)��c(OH��)��10��6����֪Kw��10��12,1 mol��L��1��NaOH��Һ����ˮ�������c(OH��)ȡ������Һ�е�c(H��)����cˮ����(OH��)��c(H��)��10��12 mol��L��1��(2)̼���Ƶ�ˮ�ⷴӦ�ֲ����У��Ե�һ��Ϊ����ǿ����������Һ�ʼ��ԣ���ˮ�������c(OH��)��c(H��)ȡ������Һ�е�c(OH��)��Ϊ10��3 mol��L��1��(3)HX��pH�仯���ȴ����С�����Խ���������ƽ�ⳣ����С��(4)ǿ�������ζ�Ӧ���������Խ������ˮ��̶Ⱦ�Խ��pHԽ������ĵ���ƽ�ⳣ��ԽС������Na2CO3��Ӧ����K2��5.6��10��11��H2CO3��K1����K(HCN)��K2С��K(HCN)�������NaCN��Һ��ͨ��������CO2������Ӧ��NaHCO3��(5)��Һ�д��ڵ���غ㣺c(CH3COO��)��c(OH��)��c(Na��)��c(H��)�����c(CH3COO��)��c(Na��)��c(H��)��c(OH��)��9.9��10��7 (mol��L��1)��ͨ������ƽ�ⳣ���ı���ʽ��֪c(CH3COO��)/c(CH3COOH)��K(CH3COOH)/c(H��)��18��

 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�