ΧβΡΩΡΎ»ί
œρ100 mL FeI2»ή“Κ÷–÷πΫΞΆ®»κCl2Θ§Μα“ά¥Έ…ζ≥…I2ΓΔFe3ΘΪΓΔIO3-Θ§Τδ÷–Fe3ΘΪΓΔI2ΒΡΈο÷ ΒΡΝΩΥφn(Cl2)ΒΡ±δΜ·»γΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ

Θ®1Θ©”…ΆΦΩ…÷ΣΘ§IΘ≠ΓΔFe2ΘΪΓΔI2»ΐ÷÷ΝΘΉ”ΒΡΜΙ‘≠–‘”…«ΩΒΫ»θΒΡΥ≥–ρΈΣ________ΘΨ________ΘΨ________ΘΜ
Θ®2Θ©Β±n(Cl2)ΘΫ0.12 mol ±Θ§»ή“Κ÷–ΒΡάκΉ”÷ς“ΣΈΣ________________________________Θ§
¥”ΩΣ ΦΆ®»κCl2ΒΫn(Cl2)ΘΫ0.12 mol ±ΒΡΉήΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________________ΘΜ
Θ®3Θ©Β±»ή“Κ÷–n(ClΘ≠)ΓΟn(IO3-)ΘΫ8ΓΟ1 ±Θ§Ά®»κΒΡCl2‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΈΣ________ΓΘ
Θ®1Θ©IΘ≠ΓΓFe2ΘΪΓΓI2
Θ®2Θ©Fe2ΘΪΓΔFe3ΘΪΓΔClΘ≠ΓΓ5FeI2ΘΪ6Cl2=5I2ΘΪ2FeCl3ΘΪ3FeCl2
Θ®3Θ©8.96 L
ΓΨΫβΈωΓΩΘ®1Θ©ΗυΨίΆΦœώΩ…÷ΣΘΚ Ήœ»IΘ≠±Μ―θΜ·Θ§»ΜΚσ «Fe2ΘΪΘ§Υυ“‘ΜΙ‘≠–‘Υ≥–ρΈΣIΘ≠ΘΨFe2ΘΪΘΨI2ΓΘΘ®2Θ©”…ΆΦœώΩ…÷Σn(I2)ΘΫ0.1 molΘ§Υυ“‘n(FeI2)ΘΫ0.1 molΘ§n(IΘ≠)ΘΫ0.2 molΓΘΒ±Ά®»κ0.12 mol Cl2Φ¥0.24 mol Cl ±Θ§IΘ≠»Ϊ≤Ω±Μ―θΜ·Θ§Fe2ΘΪ”–0.04 mol±Μ―θΜ·Θ§Υυ“‘»ή“Κ÷–ΒΡάκΉ”÷ς“Σ”–ΘΚFe2ΘΪΓΔFe3ΘΪΓΔClΘ≠Θ§ΤδΈο÷ ΒΡΝΩΖ÷±πΈΣ0.06 molΓΔ0.04 molΓΔ0.24 molΘ§
I2ΈΣ0.1 molΓΘ
n(I2)ΓΟn(FeCl3)ΓΟn(FeCl2)ΘΫ0.1ΓΟ0.04ΓΟ0.06ΘΫ5ΓΟ2ΓΟ3
ΖΫ≥Χ ΫΈΣ5FeI2ΘΪ6Cl2=5I2ΘΪ2FeCl3ΘΪ3FeCl2ΓΘ
Θ®3Θ©Fe2ΘΪ ΓΪ 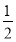 Cl2ΓΓΓΓIΘ≠ΓΓΓΪΓΓ
Cl2ΓΓΓΓIΘ≠ΓΓΓΪΓΓ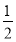 Cl2ΓΓI2ΓΪ2IO3-ΓΪ5Cl2
Cl2ΓΓI2ΓΪ2IO3-ΓΪ5Cl2
0.1 mol 0.05 mol 0.2 mol 0.1 mol x 2x 5x
”…Χβ“βΒΟΘΚ
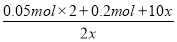 ΘΫ8
ΘΫ8
xΘΫ0.05 mol
V(Cl2)ΘΫ(0.05 molΘΪ0.1 molΘΪ5ΓΝ0.05 mol)ΓΝ22.4 LΓΛmolΘ≠1ΘΫ8.96 LΓΘ

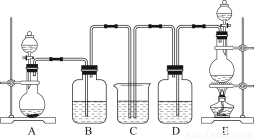
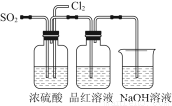
ΈΣΝΥΧΫΨΩSO2”κNa2O2ΒΡΖ¥”Π «ΖώάύΥΤ”ΎCO2”κNa2O2ΒΡΖ¥”ΠΘ§ΦΉΆ§―ß…ηΦΤΝΥ»γΆΦΥυ ΨΒΡ Β―ιΉΑ÷ΟΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ

Θ®1Θ©“ΤΩΣΟόΜ®Θ§ΫΪ¥χΜπ–«ΒΡΡΨΧθΖ≈‘ΎC ‘ΙήΩΎΘ§Έ¥ΦϊΡΨΧθΗ¥»ΦΘ§ΦΉΆ§―ß“ρ¥Υ»œΈΣSO2”κNa2O2ΒΡΖ¥”Π≤ΜΆ§”ΎCO2ΓΘ«κΑ¥ΦΉΆ§―ßΒΡΙέΒψ–¥≥ωΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®2Θ©““Ά§―ß»œΈΣΈό¬έΖ¥”Π‘≠άμ»γΚΈΘ§Ήν÷’ΕΦ”–O2≤ζ…ζΘ§““Ά§―ßΒΡάμ”… « ΓΘΑ¥’’““Ά§―ßΒΡΙέΒψΘ§ΗΟΉΑ÷Ο–ηΉωΒΡΗΡΫχ «
ΓΘ
Θ®3Θ©ΦΌ…ηNa2O2Άξ»ΪΖ¥”ΠΘ§Ζ¥”ΠΚσBΉΑ÷Ο÷–ΙΧΧε…ζ≥…ΈοΩ…Ρή «ΘΚΔΌNa2SO3ΘΜΔΎNa2SO4ΘΜΔέNa2SO3ΚΆNa2SO4ΓΘ
«κ…ηΦΤ Β―ιΖΫΑΗΦλ―ιΘ§–¥≥ω Β―ι≤Ϋ÷η“‘ΦΑ‘ΛΤΎœ÷œσΚΆΫα¬έΘ§Άξ≥…œ¬±μΘΚ
œό―Γ ‘ΦΝΘΚ2 molΓΛLΘ≠1 HCl»ή“ΚΘ§1 molΓΛLΘ≠1 HNO3»ή“ΚΘ§1 molΓΛLΘ≠1 BaCl»ή“ΚΘ§1 molΓΛLΘ≠1 BaΘ®NO3Θ©2»ή“ΚΘ§0.01 molΓΛLΘ≠1 KMnO4Υα–‘»ή“ΚΓΘ
Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσΚΆΫα¬έ |
≤Ϋ÷η1ΘΚ»ΓB÷–ΒΡ…ΌΝΩΙΧΧε―υΤΖ”Ύ ‘Ιή÷–Θ§ΒΈΦ”ΉψΝΩ’τΝσΥ°Θ§»ήΫβΘ§»ΜΚσ»Γ…ΌΝΩ¥ΐ≤β“ΚΖ÷±π÷Ο”ΎΔώΓΔΔρ ‘Ιή÷– | ΙΧΧεΆξ»Ϊ»ήΫβ |
≤Ϋ÷η2ΘΚΆυΔώ ‘Ιή÷–Φ”»κ Θ§‘ΌΒΈΦ” | Θ§ |
‘ρ÷ΛΟς…ζ≥…Έο÷–Κ§Na2SO4 |
|
≤Ϋ÷η3ΘΚΆυΔρ ‘Ιή÷– |
|
| »τ Θ§ |
‘ρ÷ΛΟς…ζ≥…Έο÷–”–Na2SO3ΘΜ»τ |
|
|
|
‘ρΥΒΟς…ζ≥…Έο÷–ΟΜ”–Na2SO3ΓΘ |
|
Θ®4Θ©…ζ≥…Έο÷–―«ΝρΥαΡΤΚ§ΝΩΒΡ≤βΕ®ΘΚ
ΔΌ»Γa g…ζ≥…Έο≈δ÷Τ≥…100 mL»ή“ΚΘ§»Γ10.00 mLΗΟ»ή“Κ”ΎΉΕ–ΈΤΩ÷–Θ§Φ”»κΦΗΒΈΒμΖέ»ή“ΚΉς÷Η ΨΦΝΘ§”Ο0.010 0 molΓΛLΘ≠1ΒβΥ°Ϋχ––ΒΈΕ®Θ§ΒΈΕ®÷’Βψœ÷œσΈΣ ΓΘΦ«¬Φ ΐΨίΘ§÷ΊΗ¥ΒΈΕ®2¥ΈΘ§ΤΫΨυœϊΚΡΒβΥ°20.00 mLΓΘ
ΔΎΦΤΥψΘΚ…ζ≥…Έο÷–―«ΝρΥαΡΤΒΡ÷ ΝΩΖ÷ ΐΈΣ ΓΘ
Υ° «…ζΟϋΒΡ‘¥»ΣΓΔΙΛ“ΒΒΡ―Σ“ΚΓΔ≥« –ΒΡΟϋ¬ωΓΘ“Σ±ΘΜΛΚΟΚ”ΝςΘ§Κ”Υ° «÷ς“ΣΒΡ“ϊ”ΟΥ°‘¥Θ§Έέ»ΨΈοΆ®Ιΐ“ϊ”ΟΥ°Ω…÷±Ϋ”ΕΨΚΠ»ΥΧεΘ§“≤Ω…Ά®Ιΐ ≥ΈοΝ¥ΚΆΙύΗ»≈©ΧοΦδΫ”ΈΘΦΑΫΓΩΒΓΘ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)¥ΩΥ°‘Ύ100 Γφ ±Θ§pHΘΫ6Θ§ΗΟΈ¬Ε»œ¬1 molΓΛLΘ≠1ΒΡNaOH»ή“Κ÷–Θ§”…Υ°Βγάκ≥ωΒΡ
c(OHΘ≠)ΘΫ________ molΓΛLΘ≠1ΓΘ
(2)25 Γφ ±Θ§œρΥ°ΒΡΒγάκΤΫΚβΧεœΒ÷–Φ”»κ…ΌΝΩΧΦΥαΡΤΙΧΧεΘ§ΒΟΒΫpHΈΣ11ΒΡ»ή“ΚΘ§ΤδΥ°ΫβΖΫ≥Χ ΫΈΣ__________Θ§”…Υ°Βγάκ≥ωΒΡc(OHΘ≠)ΘΫ__________ molΓΛLΘ≠1ΓΘ
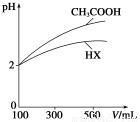
(3)ΧεΜΐΨυΈΣ100 mLΓΔpHΨυΈΣ2ΒΡCH3COOH”κ“Μ‘ΣΥαHXΘ§Φ”Υ°œΓ ΆΙΐ≥Χ÷–pH”κ»ή“ΚΧεΜΐΒΡΙΊœΒ»γ…œΆΦΥυ ΨΘ§‘ρHXΒΡΒγάκΤΫΚβ≥Θ ΐ________(ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±)CH3COOHΒΡΒγάκΤΫΚβ≥Θ ΐΓΘάμ”… «__________________________________ΓΘ
(4)ΒγάκΤΫΚβ≥Θ ΐ «ΚβΝΩ»θΒγΫβ÷ Βγάκ≥ΧΕ»«Ω»θΒΡΈοάμΝΩΓΘ“―÷ΣΘΚ
Μ·―ß Ϋ | Βγάκ≥Θ ΐ(25 Γφ) |
HCN | KΘΫ4.9ΓΝ10Θ≠10 |
CH3COOH | KΘΫ1.8ΓΝ10Θ≠5 |
H2CO3 | K1ΘΫ4.3ΓΝ10Θ≠7ΓΔK2ΘΫ5.6ΓΝ10Θ≠11 |
ΔΌ25 Γφ ±Θ§”–Β»≈®Ε»ΒΡNaCN»ή“ΚΓΔNa2CO3»ή“ΚΚΆCH3COONa»ή“ΚΘ§»ΐ»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ____________________________ΓΘ
ΔΎœρNaCN»ή“Κ÷–Ά®»κ…ΌΝΩΒΡCO2Θ§ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ__________________
(5)25 Γφ ±Θ§‘ΎCH3COOH”κCH3COONaΒΡΜλΚœ»ή“Κ÷–Θ§»τ≤βΒΟpHΘΫ6Θ§‘ρ»ή“Κ÷–c(CH3COOΘ≠)Θ≠c(NaΘΪ)ΘΫ________ molΓΛLΘ≠1(ΧνΨΪ»Ζ÷Β)Θ§c(CH3COOΘ≠)/c(CH3COOH)ΘΫ________ΓΘ