��Ŀ����
����Ŀ��
����E�ĺϳ�·�����£�
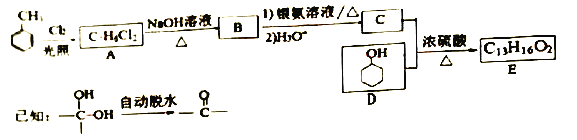
(l) B�Ļ�ѧ������____��D�й����ŵ�����Ϊ_____��
(2)C��D����E�ķ�Ӧ����Ϊ____��E�Ľṹ��ʽΪ______��
(3)1molB������������Һ��Ӧ����____g Ag��A�ĺ˴Ź���������_____���塣
(4)ͬʱ��������������C��ͬ���칹����______�֣����������칹����
����FeC13��Һ������ɫ��Ӧ ���ܷ���������Ӧ
��D�ϳ�һ�ִ���ҩI�ĺϳ�·�����£�
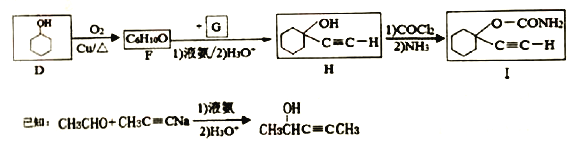
(5)D����F�Ļ�ѧ����ʽΪ______________
(6)����Ȳ�ͼ�ȩΪ��ʼԭ�ϡ�ѡ�ñ�Ҫ�����Լ��ϳ�![]() ��д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������__________��
��д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������__________��
���𰸡� ����ȩ �ǻ� ������Ӧ����ȡ����Ӧ�� ![]() 216 4 3
216 4 3 ![]()
![]()
���������ױ�����ʱ��������������ȡ������A��AΪ![]() �����������ǻ�����ͬһ��̼ԭ���ϻ��Զ���ˮ�����ʻ������A������������Һ��ˮ������B��BΪ
�����������ǻ�����ͬһ��̼ԭ���ϻ��Զ���ˮ�����ʻ������A������������Һ��ˮ������B��BΪ![]() ��B����������Ӧ����C��CΪ�����ᣬ�������뻷��������������Ӧ����E��EΪ
��B����������Ӧ����C��CΪ�����ᣬ�������뻷��������������Ӧ����E��EΪ![]() ��
��
(1)��������������BΪ![]() ����ѧ����Ϊ����ȩ��DΪ��������������Ϊ�ǻ����ʴ�Ϊ������ȩ���ǻ���
����ѧ����Ϊ����ȩ��DΪ��������������Ϊ�ǻ����ʴ�Ϊ������ȩ���ǻ���
(2)C(������)��D(������)����������Ӧ����E��E�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��������Ӧ(��ȡ����Ӧ)��
���ʴ�Ϊ��������Ӧ(��ȡ����Ӧ)��![]() ��
��
(3)1molB(![]() )������������Һ��Ӧ����2mol��������Ϊ216g��A(
)������������Һ��Ӧ����2mol��������Ϊ216g��A(![]() )����4����ԭ�ӣ��˴Ź���������4���壬�ʴ�Ϊ��216��4��
)����4����ԭ�ӣ��˴Ź���������4���壬�ʴ�Ϊ��216��4��
(4) CΪ�����ᣬ����FeC13��Һ������ɫ��Ӧ��˵���ṹ�к��з��ǻ������ܷ���������Ӧ��˵���ṹ�к���ȩ��������������C��ͬ���칹��Ϊ�����Ϻ����ǻ���ȩ��2����������3�ֽṹ���ʴ�Ϊ��3��
(5)D(������)��������������F������F�Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)����Ȳ�ͼ�ȩԭ�Ϻϳ�![]() ��������������ͼ���ϳ�
��������������ͼ���ϳ�![]() ���������Ⱥϳ�
���������Ⱥϳ�![]() �����Ը�����Ϣ
�����Ը�����Ϣ![]() ����
����![]() ���ȩ�ϳɣ���˺ϳ�·��Ϊ
���ȩ�ϳɣ���˺ϳ�·��Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ���������ƣ�NaN3����һ��Ӧ�ù㷺�Ļ�����Ʒ�������ںϳɿ�����ͷ�߾���ҩ����м��壬������ȫ���ҵȡ��ش��������⣺
��.ʵ�����Ʊ�NaN3
ˮ����(N2H4��H2O) �����������(CH3ONO)���������ƴ������Ʊ�NaN3���䷴Ӧװ����ͼ��ʾ��
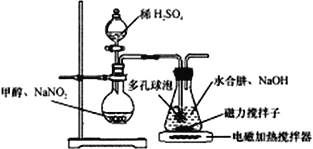
��֪��2CH3OH+2NaNO2+H2SO4��2CH3ONO+ Na2SO4+2H2O�� NaN3��ɫ��ζ�����ڴ�������ˮ
��1��N2H4�ĵ���ʽΪ_______________��NaN3�������������������Ӹ�����Ϊ______________��
��2��װ���ж�����ݵ�������___________________��
��3����ƿ��ˮ�����������������30��ʱ���Է�Ӧ���ɵ������ơ��״������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ_______________________________��
��.���ռ״�
���Ʊ���Ӧ�����û����Һ������ƿ�У�������ͼ��ʾװ�ý��м�ѹ����
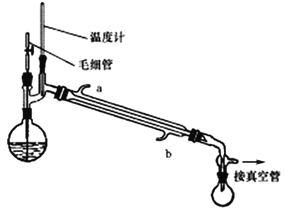
��֪��
���� | CH3OH | N2H4 | NaN3 |
�е�/�� | 64.7 | 113.5 | 300 |
NaN3��40��ʱ�ֽ�
��4��ʵ��ʱ����������ȴˮҪ��b��a����ԭ����______________________��
��5���״�����ʱ���ü�ѹ�����ԭ����________________________��
��6�������й�ëϸ�ܵ�����˵����ȷ����_______________��
A.ƽ��Բ����ƿ����ѹ B.��Ϊ�������ģ�ʹ����ƽ��
C.����Һ����ȶ����� D.������������
��.��Ʒ��ȡ�����Ȳⶨ
�����������ĸҺ���½ᾧ�����˵�NaN3ʪƷ������ȥ����ˮ�ؽᾧ��NaN3��Ʒ���õ������ⶨ��Ʒ���ȡ�ȡ��Ʒ6.50g��������ȥ����ˮ���ܽ⣬����������ϡ�����ữ������Һ�м���20.00mL 1.00mol��L-lKMnO4��Һ����Һ���Ϻ�ɫ���ټ�������KI��Һ���Ĺ�����KMnO4��Һ�������0.100mol��L-lNa2S2O3����Һ�ζ���������I2������Na2S2O3��Һ30.00mL��
��7��ʵ�����ò�Ʒ�Ĵ���Ϊ______________________��
��֪���ٲ�Ʒ�����ʲ����뷴Ӧ��
�ڲⶨ�����з����ķ�Ӧ��
10NaN3+2KMnO4+8H2SO4==2MnSO4+K2SO4+5Na2SO4+8H2O+15N2����
10KI+2KMnO4+8H2SO4==2MnSO4+6K2SO4+8H2O+5I2��
I2+2Na2S2O3==2NaI +Na2S4O6��


