��Ŀ����
A��B��C��D��E�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ�����4����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���壮
��1��D�����ڱ��е�λ�� ��B��ԭ�Ӻ�������Ų�ʾ��ͼ ��
��2��EԪ���γ�����������Ӧˮ����Ļ�ѧʽΪ ��
��3��Ԫ��C��D��E�γɵ�ԭ�Ӱ뾶��С��ϵ�� ����Ԫ�ط��ű�ʾ����
��4��C��D���γɻ�����D2C2��D2C2���еĻ�ѧ���� ��
��5��A��C����Ԫ���γɵ�ԭ�Ӹ���֮��Ϊ1:1�Ļ��������ʽ ��
��6��B���⻯����B������������ˮ���ﷴӦ�����ӷ���ʽ ��
��1���������ڵڢ�A�� ��2�֣� ��2�֣�
��2�֣�
��2��H2SO4 ��2�֣�
��3��Na > S > O (2�֣�
��4�����Ӽ������ۼ� ��2�֣�
��5�� ��2�֣�
��2�֣�
��6��NH3 + H+ = NH4+ ��2�֣�
���������������1��AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�A��HԪ�أ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��BΪ��������Ԫ�أ���ԭ�Ӱ뾶��ͬ������С������B��NԪ�أ�CԪ��ԭ�ӵ������������ȴ�����4����˵��Cֻ����2����ӣ�����C��OԪ�أ�C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��DΪ+1��Ԫ�أ�����D��Na��C��Eͬ���壬��E��SԪ�أ�����Na��Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壻N��ԭ�Ӻ�������Ų�ʾ��ͼ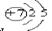 ��
��
��2��SԪ���γ�����������Ӧˮ����Ļ�ѧʽΪH2SO4��
��3������ԭ�Ӱ뾶�Ĵ�С���ɣ�Ԫ��O��Na��S�γɵ�ԭ�Ӱ뾶��С��ϵ��Na > S > O��
��4�����������м������Ӽ������й��ۼ���
��5��A��C����Ԫ���γɵ�ԭ�Ӹ���֮��Ϊ1:1�Ļ�����Ϊ�������⣬�ǹ��ۻ��������ʽΪ ��
��
��6��B���⻯���ǰ�������B������������ˮ�������ᷴӦ��������泥����ӷ���ʽΪNH3 + H+ = NH4+��
���㣺����Ԫ���ƶϣ�Ԫ����Ԫ�����ڱ���λ�õ��жϣ�����ʽ��ԭ�ӽṹʾ��ͼ�����ӷ���ʽ����ѧʽ����д����ѧ�����жϣ�ԭ�Ӱ뾶�ıȽ�

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�[���ʽṹ�����ʣ�13��]
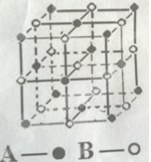
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ ��
��2����ͼ���߱�ʾ���ֶ�����Ԫ�ص�ԭ��������������˳�����У����䳣�����ʷе�Ĺ�ϵ������A���ʾ�ĵ����� ���ѧʽ����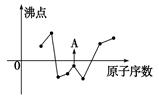
| ����/(pm) | B��F | B��Cl | B��Br |
| ����ֵ | 152 | 187 | 199 |
| ʵ��ֵ | 130 | 175 | 187 |
��4������Ʒ���Ӽ��������������Na+���������������ɵģ�ij�ֶ��������Ľṹ��ͼ��
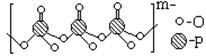
����ԭ�ӵ��ӻ�����Ϊ ��
�����ֶ�������ƵĻ�ѧʽΪ ��
��5����֪HF��F��ͨ�������ϳ�HF
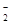 ���ж�HF
���ж�HF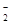 ��HF
��HF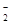 �����ܷ��γ��������˵�����ɡ�
�����ܷ��γ��������˵�����ɡ���
��15�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
| A | A������������Ӧ��ˮ���ﻯѧʽΪH2AO3 |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | Cԭ����ͬ����ԭ���а뾶���ϡ��������⣩���䵥����ɫΪ��ɫ |
| D | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p2 |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��Ϊ��������������������A������Ȼ��ﹹ�ɾ��������������Ϊ������������������������������
��3��A��B������⻯���н��ȶ����������������ѧʽ����B������⻯���E�ĺ�ɫ����������ڼ���ʱ�ɷ�Ӧ��д���䷴Ӧ����ʽ������������������������������������
��4)E�ĵ��ʺ���������ϡ�����пɷ�Ӧ�����˽������Ӧ��Ƴ�ԭ��أ���д��������Ӧ����ʽ��������������������������������������������
��5��úȼ�ղ�������������B����������������صĻ������⣬��ˣ�����AH4����ԭ��������Ⱦ����֪��
�� AH4(g)+2 BO2��g)�� B2(g)+AO2(g)+2H2O (g) ��H1����867kJ��mol
�� 2BO2(g) ?B2O4(g) ��H2=��56.9 kJ��mol
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽ������������������������������������������������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺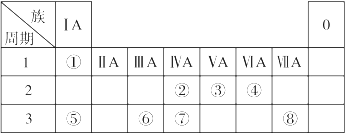
��1��������Ԫ�ص�ԭ���У�ԭ�Ӱ뾶������ ����Ԫ�ط��ţ���
��2���ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ���� ��
��3���١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д�����ֻ�����ĵ���ʽ �� ��
��4��W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е������� ��
| A����������ϼ�Ϊ��6�� | B����̬�⻯���H2S�ȶ� |
| C������������ˮ��������Ա������� | D�������ڳ����¿����������� |
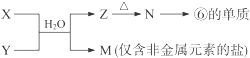
X��Һ��Y��Һ��Ӧ�����ӷ���ʽ ��
M�������ӵļ������� ��
��14�֣��±�ΪԪ�����ڱ���һ���֣�
 �� ������ | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
��1��д��Ԫ�آ������ڱ��е�λ�ã��������� ������
��2���ڢۢݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ�������� ������
��3���ܢݢ���̬�⻯����ȶ�����ǿ������˳���������� ����
��4���٢ڢۢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д���������ֻ�
����ĵ���ʽ�������� ��������
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ�еı仯������A��һ
�ֵ���ɫ���塣��
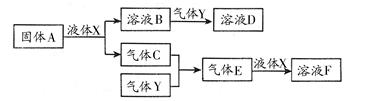
��1��д������A��Һ��X��Ӧ�����ӷ���ʽ�������������� ����������
��2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1��1��ǡ����ȫ��Ӧʱ��������ҺD������Ϊ���� �������ѧʽ������֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ ����
��3����100 mL 18 mol/L��FŨ��Һ�м������ͭƬ������ʹ֮��ַ�Ӧ��������������������£�����Ϊ��������������������
A��40.32 L B��30.24 L C��20.16 L D��13.44 L
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��1������ЩԪ����,��ѧ��������õ���: (����廯ѧ����,��ͬ)��
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����ĵ���ʽ�ǣ� ��
��3������������������������Ԫ���� ��д���������������������Ʒ�Ӧ�����ӷ���ʽ ��
��4�����⻯����۵ĵ�����һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��5�� �ڿ����γɶ������������һ���Ǻ���ɫ���壬���÷���ʽ˵�������岻�˲�����ˮ���ռ���ԭ�� ��
��6�� �ýṹʽ��ʾԪ�آ�����γɵĻ����� ���û������ڹ���ʱ�׳� ������ ���壬ָ������һ����; ��
�±�ΪԪ�����ڱ���һ���֣�
| �� ���� | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
�������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1��д��Ԫ�آڵ����ӽṹʾ��ͼ______________��
��2���ڡ��ۡ��ݵ����Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��3��Ԫ�آ�����γɻ�����ĵ���ʽ��_________________________��
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ��ʾ�ı仯������A��һ�ֵ���ɫ���塣��ش�
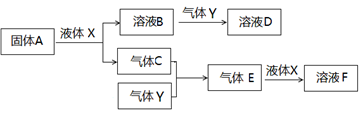
��4��д������A��Һ��X��Ӧ�����ӷ���ʽ ��
��5������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1:1��ǡ����ȫ��Ӧʱ��������ҺD����֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
��6����500�棬101kPaʱ������C������Y��Ӧ����0��2mol����Eʱ���ų�akJ������д���������·�Ӧ���Ȼ�ѧ����ʽ ��
��7��������C��Y�ں��ݾ��ȵ������·�Ӧ������˵�����жϴﵽƽ��״̬���� ��
A���¶Ȳ��� B��������ѹǿ���� C�����������ܶȲ��� D����������ƽ������������
��֪X��Y��ZΪͬһ�����ڵ�����Ԫ�أ���ԭ�ӵIJ��ֵ�����(kJ��mol��1)���±���ʾ��
| | X | Y | Z |
| I1 | 496 | 738 | 578 |
| I2 | 4562 | 1451 | 1817 |
| I3 | 6912 | 7733 | 2745 |
| I4 | 9543 | 10540 | 11575 |
(1)����Ԫ�ص縺�Դ�С��ϵΪ ��
(2)д��Yԭ�ӵĵ����Ų�ʽ ��Y�ĵ�һ�����ܴ���Z�ĵ�һ�����ܵ�ԭ�� ��
(3)X����������� (����ĸ)�ѻ���ʽ��
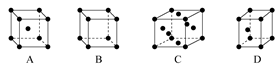
(4)����Ԫ��X���ε���ɫ��ӦΪ ɫ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
(5)NaCl��KCl��MgO��CaO����ṹ���ƣ��������־���ľ������������±���
| ���� | NaCl | KCl | CaO |
| ������/(kJ��mol��1) | 786 | 715 | 3401 |
4�־���NaCl��KCl��MgO��CaO�۵��ɸߵ��͵�˳���� ��