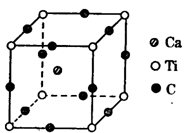
��Ŀ����
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��1������ЩԪ����,��ѧ��������õ���: (����廯ѧ����,��ͬ)��
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����ĵ���ʽ�ǣ� ��
��3������������������������Ԫ���� ��д���������������������Ʒ�Ӧ�����ӷ���ʽ ��
��4�����⻯����۵ĵ�����һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��5�� �ڿ����γɶ������������һ���Ǻ���ɫ���壬���÷���ʽ˵�������岻�˲�����ˮ���ռ���ԭ�� ��
��6�� �ýṹʽ��ʾԪ�آ�����γɵĻ����� ���û������ڹ���ʱ�׳� ������ ���壬ָ������һ����; ��
��1��Ar
��2��HClO4��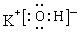
��3��Al��Al2O3+2OH-��2AlO2-+H2O
��4��4NH3��5O2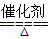 4NO��6H2O��4NH3��3O2
4NO��6H2O��4NH3��3O2 2N2��6H2O
2N2��6H2O
��5��3NO2��H2O=2HNO3��NO
��6��O��C��O���ɱ������ӣ��˹�����
�����������������Ԫ�������ڱ��еķֲ���������֪����C������N������O������Na������Al������S������Cl������Ar������K����
��1������ЩԪ���У���ѧ�������ȶ�����ϡ������Ar��
��2������Ԫ�������ɣ�ͬ����Ԫ�ص�ԭ�ӣ�����������������Ӧˮ�������������ǿ������������ͬ����Ԫ�ص�ԭ�ӣ����µ�������������Ӧˮ�����������������������ǿ������֪������ǿ�ĺ������Ǹ����ᣬ������ǿ����KOH���������������ӻ���������ʽ�ɱ�ʾΪ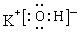 ��
��
��3�����������������������������������������������Ԫ����Al���������ܺ�ǿ�Ӧ�����κ�ˮ�����ӷ���ʽΪAl2O3+2OH-��2AlO2-+H2O��
��4�����⻯���ǰ�����۵ĵ���������һ�������·�Ӧ�Ļ�ѧ����ʽΪ��4NH3��5O2 4NO��6H2O��4NH3��3O2
4NO��6H2O��4NH3��3O2 2N2��6H2O��
2N2��6H2O��
��5������ɫ������NO2��NO2����ˮ����NO�����ᣬ��������ˮ���ռ�����Ӧ�Ļ�ѧ����ʽΪ3NO2��H2O=2HNO3��NO
��6��̼�������ɵĻ�������CO2���ṹʽΪO��C��O��CO2�ڹ�̬ʱ�׳Ƹɱ����γɵľ����Ƿ��Ӿ��壬���������˹����ꡣ
���㣺����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
 ����
���� ������
������