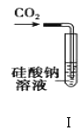
��Ŀ����
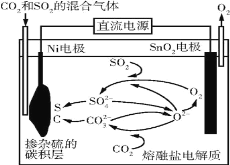
����Ŀ�������������������ܺ����Ա��㷺Ӧ�������ࡢ���ӡ������ȹ�ҵ���϶�ӰҺ������Ҫ��Na3Ag(S2O3)2��ʽ���ڣ�ʵ�����÷϶�ӰҺ�Ʊ�Ag�ľ���������ͼ��ʾ��
ע�⣺����ԭ��ʱ����Ag+ֱ����N2H4H2O��Ӧ���ڼ��ң����Բ��ü��백ˮ��ʹAg+�백�γ�[Ag(NH3)2]+������Ag+��Ũ�ȣ��Ӷ���Ӧ����Ag+������������ʹ��Ӧ�ܹ�ƽ�Ƚ��С�
�ش��������⣺
��1����������ʱ������������__(�����ʵ����ƣ����õ�1mol������ת�Ƶ���Ϊ__mol��
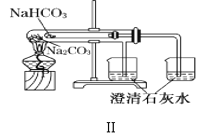
��2��N2H4H2O(ˮ���£�Ϊ��ɫ������״����Һ�壬����ǿ��ԭ�ԣ�ʵ�����Ʊ�ԭ��ΪNaClO+2NH3=N2H4H2O+NaCl�������õ���ʵ��װ����ͼ��ʾ��
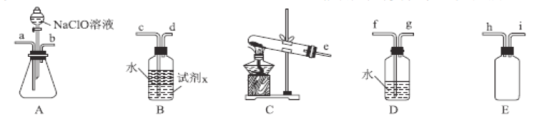
�ٱ�ʵ�����ò�����װ����__(����ĸ���������������ҵķ�������װ�õ�����˳��Ϊ__(�������ӿ�Сд��ĸ����
�ڴ�������ԭ�Ƕȷ�����������Ӧ��NaClO���ֳ�������Ϊ__������NaClO��ҺʱҪ�����μӣ�Ŀ����__��
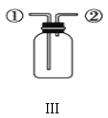
��3��AgNO3����������ֽ⡣������������AgNO3��Һ����Ũ���ɻ��AgNO3���壬ʵ��װ����ͼ��ʾ��

��ʹ����ձõ�Ŀ����__��
�ڲⶨAgNO3����Ĵ���(���ʲ����뷴Ӧ����ȡ2.000g�Ʊ���AgNO3���壬��ˮ�ܽ⣬���ݵ�100mL��ȷ��ȡ25.00mL��Һ���ữ����뼸��NH4Fe(SO4)2��Һ��ָʾ��������0.1000molL-1NH4SCN����Һ�ζ�������NH4SCN����Һ��ƽ�����Ϊ29.00mL���������AgNO3����������Ϊ__��
���𰸡�һ������ 3 D ehi(��ih)abc(d) ��ǿ�������� ˮ���»�ԭ�Ժ�ǿ����ֹNaClOŨ�ȹ��������� ʹ��ϵ�γɸ�ѹ���Ӷ�����ˮ���ڽϵ͵��¶���������ͬʱ����AgNO3�ֽ� 98.6%
��������
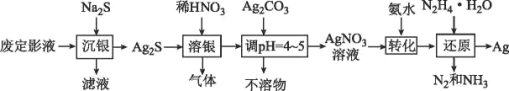
��������ͼ��֪���϶�ӰҺ�м���Na2S�õ�Ag2S���������˺�õ�Ag2S������ʱ����ϡ���ᷢ����Ӧ3Ag2S+ 8HNO3= 6AgNO3+ 2NO��+3S+ 4H2O�����Եõ���������NO������Һ�м���Ag2CO3������Һ��pH��4~ 5��Ȼ����ˣ��õ�AgNO3��Һ������Һ�м��백ˮ���õ�[Ag(NH3)2]+��Ȼ������·���������ԭ��Ӧ4[Ag(NH3)2]++ N2H4H2O=4Ag+N2��+8NH3��+H2O+4H+��
��1����������ͼ����������������Ag2S�м���ϡ���ᣬAg2S�е���Ϊ-2�ۣ�������ͼ�̬�����л�ԭ�ԣ�������������ԣ����߷���������ԭ��Ӧ��Ag2S�е��ϼ�����ת��Ϊ���ʣ�ϡ������������ԭΪNO����Ԫ�ػ��ϼ۸ı�3�ۣ����������������в���������Ϊһ������NO���õ�1mol������ת�Ƶ���Ϊ3mol��
��2���ٸ����Ʊ�N2H4H2O�ķ�ӦNaClO+2NH3=N2H4H2O+NaCl��֪����Ҫ�Ʊ�������ʵ�������Ȼ�狀��������ƹ��������ȡ��������Ӧѡ��װ��C������װ��A�з�Һ©���е�ҩƷΪNaClO����Aװ��Ϊ��ȡˮ���µ�װ�ã�װ��E��ȫƿ���ã�������������ˮ������ʹ��װ��D���ɴ˵ó�����������ҵķ���װ�õ�����˳��Ϊehi(ih)abc(d)��
��N2H4H2O����ǿ��ԭ�ԣ�NaClO���������ԣ���NaClO��Һ������쵼�¹������ɽ���Ӧ���ɵ�N2H4H2O�������������μ�NaClO��Һ�ܷ�ֹ���ɵ�N2H4H2O��������
��3���ٸ�������ṩ��Ϣ��AgNO3����������ֽ⣬ʵ��װ������ձÿ����γɸ�ѹ��������ˮ���ڽϵ͵��¶���������ͬʱ��ֹAgNO3�ֽ⣻
��NH4SCN����Һ��ƽ�����Ϊ29.00mL����NH4SCN���ʵ���Ϊ0.1000molL1��0.029L
=2.9��103mol�����������غ㣬��Ϸ�ӦAg++SCN=AgSCN����֪��������������Ϊ2.9��103mol��170g/mol��![]() =1.972g����������������������Ϊ
=1.972g����������������������Ϊ![]() ��100%=98.60%��
��100%=98.60%��

����Ŀ��![]() �ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ
�ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ![]() ������
������![]() �����ʣ��Ʊ������ֹ����������£�
�����ʣ��Ʊ������ֹ����������£�
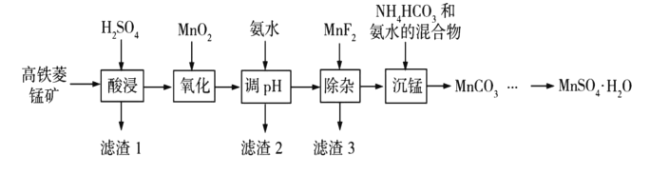
��ؽ������������������������![]() �������ʼ������
�������ʼ������![]() ������Ũ��Ϊ
������Ũ��Ϊ![]() ���㣩��
���㣩��
�������� |
|
|
|
|
|
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 |
��1����������ʱ������Ӧ�����ӷ���ʽΪ___________��
��2������pH����Χ��5~6���õ�����2����Ҫ�ɷֳ�![]() ���___________��
���___________��
��3�������ӡ������м���![]() ��Ŀ����___________��
��Ŀ����___________��
��4�������̡������з�����Ӧ�Ļ�ѧ����ʽΪ___________��
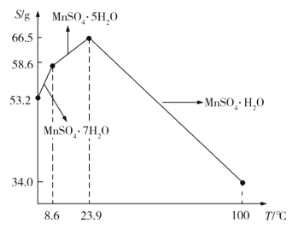
��5��![]() ��ˮ�е��ܽ�����¶ȵĹ�ϵ��ͼ��ʾ����
��ˮ�е��ܽ�����¶ȵĹ�ϵ��ͼ��ʾ����![]() ��ýϴ�����
��ýϴ�����![]() ����ķ����ǣ���
����ķ����ǣ���![]() ����������ϡ���ᣬ�����¶���80��~90��֮�������ᾧ��__________����������ƣ����õ�
����������ϡ���ᣬ�����¶���80��~90��֮�������ᾧ��__________����������ƣ����õ�![]() ���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����__________��
���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����__________��
��6����֪��![]() �������£�����Һ��
�������£�����Һ��![]() ����ʹ��Һ�е�
����ʹ��Һ�е�![]() �����������ҺpH��ΧΪ______________________��
�����������ҺpH��ΧΪ______________________��
����Ŀ��������X����Y��Һ�У����ɳ������ʵ���n2������X�����ʵ���n1�Ĺ�ϵ��ͼ��ʾ������ͼ��ʾ�������
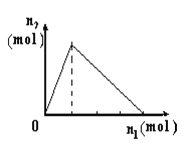
A | B | C | D | |
X | NaOH | AlCl3 | HCl | NaAlO2 |
Y | AlCl3 | NaOH | NaAlO2 | HCl |
A. A B. B C. C D. D