��Ŀ����
����Ŀ��![]() �ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ
�ڹ�ҵ��ũҵ�ȷ����й㷺��Ӧ�ã���ҵ�Ͽ��ɸ������̿���Ҫ�ɷ�Ϊ![]() ������
������![]() �����ʣ��Ʊ������ֹ����������£�
�����ʣ��Ʊ������ֹ����������£�
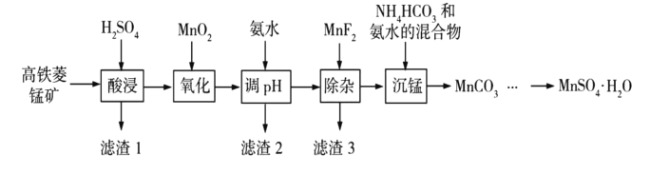
��ؽ������������������������![]() �������ʼ������
�������ʼ������![]() ������Ũ��Ϊ
������Ũ��Ϊ![]() ���㣩��
���㣩��
�������� |
|
|
|
|
|
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 |
��1����������ʱ������Ӧ�����ӷ���ʽΪ___________��
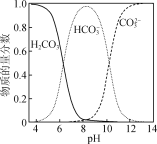
��2������pH����Χ��5~6���õ�����2����Ҫ�ɷֳ�![]() ���___________��
���___________��
��3�������ӡ������м���![]() ��Ŀ����___________��
��Ŀ����___________��
��4�������̡������з�����Ӧ�Ļ�ѧ����ʽΪ___________��
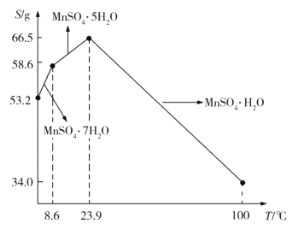
��5��![]() ��ˮ�е��ܽ�����¶ȵĹ�ϵ��ͼ��ʾ����
��ˮ�е��ܽ�����¶ȵĹ�ϵ��ͼ��ʾ����![]() ��ýϴ�����
��ýϴ�����![]() ����ķ����ǣ���
����ķ����ǣ���![]() ����������ϡ���ᣬ�����¶���80��~90��֮�������ᾧ��__________����������ƣ����õ�
����������ϡ���ᣬ�����¶���80��~90��֮�������ᾧ��__________����������ƣ����õ�![]() ���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����__________��
���壬ϴ�ӡ���ɡ�����ͨ�����ü�ѹ��ɵ�ԭ����__________��
��6����֪��![]() �������£�����Һ��
�������£�����Һ��![]() ����ʹ��Һ�е�
����ʹ��Һ�е�![]() �����������ҺpH��ΧΪ______________________��
�����������ҺpH��ΧΪ______________________��
���𰸡�![]()
![]() ��ȥ
��ȥ![]()
![]() ���ȹ��� ��ֹ
���ȹ��� ��ֹ![]() ʧȥ�ᾧˮ 3~9
ʧȥ�ᾧˮ 3~9
��������
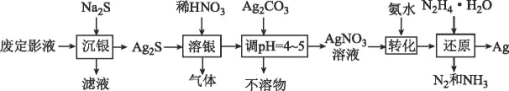
�������̿���Ҫ�ɷ�Ϊ![]() ������
������![]() �����ʣ�����������ܽ�ʱ���������費�������ᣬ���������������̼���ξ���������ת�����Ӧ�������Σ����������м���������̽������������������������ӣ�Ȼ��Ӱ�ˮ������Һ��pHֵ����������ת����������������ȥ�����˺���Һ��MnF2����þ���ӣ����˺�����Һ�м�̼����狀Ͱ�ˮ����ォ������ת���̼���̳�����Ȼ����˺�̼��������������ϡ����õ������̵���Һ���ھ����ᾧ�õ�
�����ʣ�����������ܽ�ʱ���������費�������ᣬ���������������̼���ξ���������ת�����Ӧ�������Σ����������м���������̽������������������������ӣ�Ȼ��Ӱ�ˮ������Һ��pHֵ����������ת����������������ȥ�����˺���Һ��MnF2����þ���ӣ����˺�����Һ�м�̼����狀Ͱ�ˮ����ォ������ת���̼���̳�����Ȼ����˺�̼��������������ϡ����õ������̵���Һ���ھ����ᾧ�õ�![]() ���壬�ݴ˽��
���壬�ݴ˽��
��1����������ʱ�������̽������������������������ӣ����ӷ�ӦΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2������pH����Χ��5~6���õ�����2�����й�����![]() �⣬�������������Ӻ�������ˮ��õ����������������������������ʴ�Ϊ��
�⣬�������������Ӻ�������ˮ��õ����������������������������ʴ�Ϊ��![]() ��
��
��3��������Ϸ��������ӡ���������Һ�н���þ����һ�������������ʣ���˼���![]() ��Ŀ���ǽ�þ����ת��ɷ���þ������ȥ���ʴ�Ϊ����ȥ
��Ŀ���ǽ�þ����ת��ɷ���þ������ȥ���ʴ�Ϊ����ȥ![]() ��
��
��4�������̡���������Һ�е���������̼����狀Ͱ�ˮ��Ӧ����̼���̳���������泥���Ӧ�ķ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5����![]() ��ýϴ�����
��ýϴ�����![]() ����ķ����ǣ���
����ķ����ǣ���![]() ����������ϡ���ᣬ�õ���������Һ�������ܽ�����߿�֪
����������ϡ���ᣬ�õ���������Һ�������ܽ�����߿�֪![]() ��
��![]() ����ʱ���¶ȵ������ܽ����С����˿����¶���80��~90��֮�������ᾧ��Ȼ��Ҫ���ȹ����Լ���
����ʱ���¶ȵ������ܽ����С����˿����¶���80��~90��֮�������ᾧ��Ȼ��Ҫ���ȹ����Լ���![]() ������ܽ���ʧ���õ���������ϴ�ӡ���ɣ�ͨ�����ü�ѹ����������Խ���Һ��ķе�������ˮ�ڵ���ʱ�ӷ����Է��¶ȹ���ʱ���¾������ȷֽ�ʧȥ�ᾧˮ���ʴ�Ϊ�����ȹ��ˣ���ֹ
������ܽ���ʧ���õ���������ϴ�ӡ���ɣ�ͨ�����ü�ѹ����������Խ���Һ��ķе�������ˮ�ڵ���ʱ�ӷ����Է��¶ȹ���ʱ���¾������ȷֽ�ʧȥ�ᾧˮ���ʴ�Ϊ�����ȹ��ˣ���ֹ![]() ʧȥ�ᾧˮ��
ʧȥ�ᾧˮ��
��6����֪��![]() ����ʹ��Һ�е�
����ʹ��Һ�е�![]() ��
��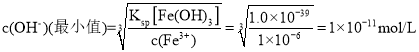 ����ʱ��pHֵΪ3��ͬʱ�ֲ�ʹþ���ӳ�����
����ʱ��pHֵΪ3��ͬʱ�ֲ�ʹþ���ӳ�����![]() ����
����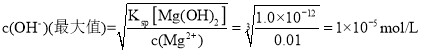 ,��ʱpHֵΪ9�����pHֵ�ķ�ΧΪ��3~9���ʴ�Ϊ�� 3~9��
,��ʱpHֵΪ9�����pHֵ�ķ�ΧΪ��3~9���ʴ�Ϊ�� 3~9��

����Ŀ��ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֡�ij��ѧ��ȤС��Ϊ��֤���������ĩ����ɣ���������ʵ�飺
(1)��ͬѧ����1mol/L�����ᡢKSCN��Һ�����Ը��������Һ��ȷ�������
��� | ʵ����� | ʵ����������� |
�� | ȡ������ĩ�����Թ��У�ע��1mol/L������ | ��ĩ���ܽ⣬��Һ�ʻ���ɫ |
�� | ������������Һ�ֳ����ݣ�������һ�ݵμӼ���__________���� | ����Һ________ ��˵����Fe2O3���� |
�� | ����һ����Һ�м�������__________���� | ����Һ________ ��˵����FeO���� |
(2)��ͬѧ��ȡ30.4g���壬�����²������ʵ�飺
����һ �����������ձ��У�����1mol/L�����Ὣ������ȫ�ܽ�
����� ���ձ����ȼ�����������ˮ����ַ�Ӧ���ټ�������������������Һ
������ ����������õ����ʹ���ϴ�Ӻ������������������ٱ仯���õ�32g����ɫ����
�ٲ�����м�����ˮ��Ŀ����__________________________________________��
�ڲ������г���ϴ�ӵIJ���Ϊ__________________________________________��
��ԭ������FeO��Fe2O3�����ʵ���֮��Ϊ___________________��