��Ŀ����
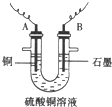
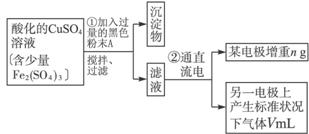
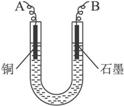
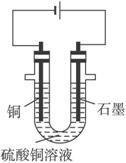
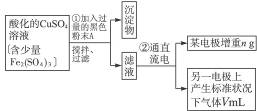
��֪��pHΪ4-5�Ļ����У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ�ⴿ����CuSO4��Һ�ķ��������ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ����������ʵ���������ͼ��ʾ��
��1������������A������______
a��NH3?H2O b��Cu c��CuO d��Cu��OH��2
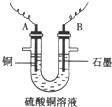
��2�����˲������õ��IJ���������______������������õIJ���������ͼ����A����ֱ����Դ��______����B�缫�Ϸ����ĵ缫��ӦΪ______��
��3����ʼһ��ʱ�����U�ι��й۲쵽��������______�������ӷ���ʽΪ______��
��4������ʵ������б�Ҫ���ǣ�����ĸ��______��
A���������ǰ�缫������
B�����缫�ں�ɳ���ǰ������������ˮ��ϴ
C�����µ���缫��������ͭ������ϴ������
D���缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء�����
E�����п������ڵ�����£���ɵ缫������õ��º�ɵķ���
��5��ͭ�����ԭ������Ϊ______���ô���n��V�ļ���ʽ��ʾ����

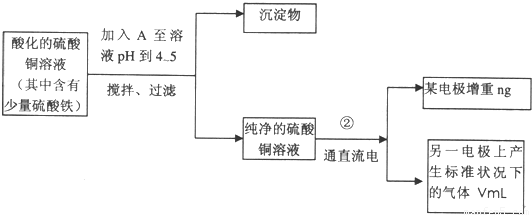
��1������������A������______
a��NH3?H2O b��Cu c��CuO d��Cu��OH��2

��2�����˲������õ��IJ���������______������������õIJ���������ͼ����A����ֱ����Դ��______����B�缫�Ϸ����ĵ缫��ӦΪ______��
��3����ʼһ��ʱ�����U�ι��й۲쵽��������______�������ӷ���ʽΪ______��
��4������ʵ������б�Ҫ���ǣ�����ĸ��______��
A���������ǰ�缫������
B�����缫�ں�ɳ���ǰ������������ˮ��ϴ
C�����µ���缫��������ͭ������ϴ������
D���缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء�����
E�����п������ڵ�����£���ɵ缫������õ��º�ɵķ���
��5��ͭ�����ԭ������Ϊ______���ô���n��V�ļ���ʽ��ʾ����
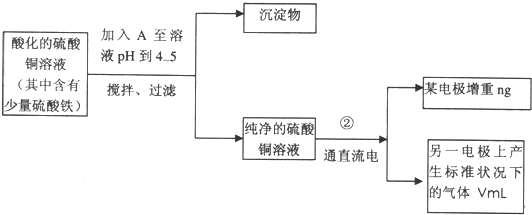
��1��������ˮ�⣬����pH��ʹ������ת��Ϊ��������ȥ��Cu�������Ӳ���Ӧ���Ӱ�ˮ���������ʣ�ѡcd����������ˮ��ƽ�������ƶ�����ȫת��Ϊ������
�ʴ�Ϊ��cd��
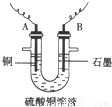
��2��������Ҫ©�����ձ�����������������A������Cu����AΪ���������Դ����������BΪ������������ӦΪ40H--4e-�T02��+2H20��
�ʴ�Ϊ���ձ�����������©�������� 40H--4e-�T02��+2H20��
��3����ⷢ��2Cu2++2H20
2Cu+4H++02�����۲쵽ͭ����֣�ʯī�������������ɣ���Һ����ɫ��dz��
�ʴ�Ϊ��ͭ����֣�ʯī�������������ɣ���Һ����ɫ��dz��2Cu2++2H20
2Cu+4H++02����
��4�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
A��ʵ��֮ǰӦ�������ǰ�缫������������ȷ
B�����缫�ں�ɳ���ǰ������������ˮ��ϴ������������ȷ��
C�����µ���缫��������ͭ������ϴ�����أ���������ȷ���ѵõ�ȷ��Cu���������ʴ���
D���缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء����У���ֹCu������������ȷ��
E�����п������ڵ�����£���ɵ缫������õ��º�ɵķ�������ֹCu������������ȷ��
�ʴ�Ϊ��ABDE��
��5����Cu�����ԭ������Ϊx��
���ݵ����غ��֪��2Cu��02������
2x 1
ng
2x��
=n��
���x=
���ʴ�Ϊ��
��
�ʴ�Ϊ��cd��
��2��������Ҫ©�����ձ�����������������A������Cu����AΪ���������Դ����������BΪ������������ӦΪ40H--4e-�T02��+2H20��
�ʴ�Ϊ���ձ�����������©�������� 40H--4e-�T02��+2H20��
��3����ⷢ��2Cu2++2H20
| ||
�ʴ�Ϊ��ͭ����֣�ʯī�������������ɣ���Һ����ɫ��dz��2Cu2++2H20
| ||
��4�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
A��ʵ��֮ǰӦ�������ǰ�缫������������ȷ
B�����缫�ں�ɳ���ǰ������������ˮ��ϴ������������ȷ��
C�����µ���缫��������ͭ������ϴ�����أ���������ȷ���ѵõ�ȷ��Cu���������ʴ���
D���缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء����У���ֹCu������������ȷ��
E�����п������ڵ�����£���ɵ缫������õ��º�ɵķ�������ֹCu������������ȷ��
�ʴ�Ϊ��ABDE��
��5����Cu�����ԭ������Ϊx��
���ݵ����غ��֪��2Cu��02������
2x 1
ng
| V��10-3L |
| 22.4L/mol |
2x��
| V��10-3L |
| 22.4L/mol |
���x=
| 11200n |
| V |
| 11200n |
| V |

��ϰ��ϵ�д�
�����Ŀ