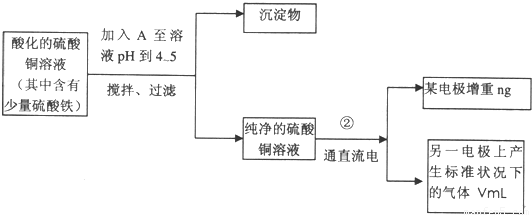
��Ŀ����
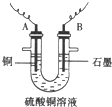
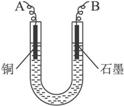
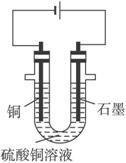
��֪��pHΪ4��5�Ļ����У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ�����õ�ⴿ��CuSO4��Һ�ķ����������ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ����������ʵ���������£�
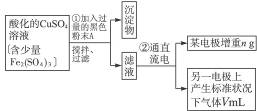

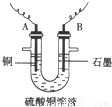
ͼ4-16
�Իش��������⣺
(1)������������A�Ļ�ѧʽΪ________________������A��������___________________,
(2)����������ò���������ͼ4-16��ʾ����AӦ��ֱ����Դ��__________����
(3)��ʼ��һ��ʱ�䣬��U�ι��ڿɹ۲쵽��������____________�������ܷ�Ӧ�����ӷ���ʽΪ________________________��
(4)����ʵ���������Ҫ����__________(����ĸ����ͬ)��������Ҫ����________________��
A.�������ǰ�缫������
B.����缫�ں�ɡ�����ǰ������������ˮ��ϴ
C.���µ���缫�ϵ�ͭ������ϴ������
D.�缫�ں�ɳ����IJ������밴��ɡ��������ٺ�ɡ��ٳ�����������
E.���п������ڵ�����£��缫��ɱ�����õ��º�ɷ�
(5)ͭ�����ԭ������Ϊ______________________��
(1)CuO ����H+ʹ��Һ��pH���ߣ�Fe3+ˮ������Fe(OH)3���� (2)��
(3)��B������ɫ��ζ�������ɣ���A���к�ɫ�������ɣ��������Һ����ɫ��dz2Cu2++2H2O![]() 2Cu+4H++O2�� (4)ABDE C (5)
2Cu+4H++O2�� (4)ABDE C (5)![]()
����:
2Cu2++2H2O![]() 2Cu��4H++O2����
2Cu��4H++O2����
n(Cu)��n(O2)=2��1��n(Cu)=![]() mol,n(O2)=
mol,n(O2)=![]()
![]()
![]()