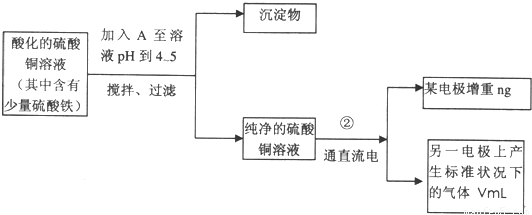
��Ŀ����
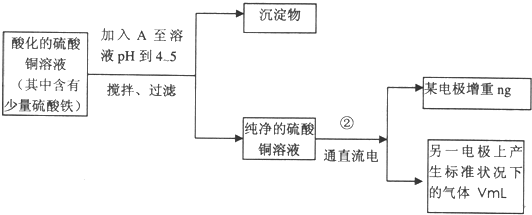
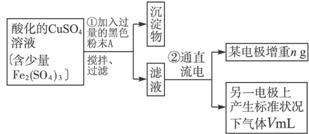
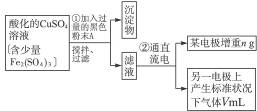
��֪��pHΪ4��5�Ļ����У�Cu2+������ˮ�⣬��Fe3+����ȫˮ�⣬ת��Ϊ Fe��OH��3��ijͬѧ���ᴿ��������Fe2��SO4��3��ϡH2SO4��CuSO4��Һ�������õ�ⴿ����CuSO4��Һ�õ������ݣ�����Cu�����ԭ��������
��1����������Fe2��SO4��3��ϡH2SO4��CuSO4��Һ�У������Թ����ĺ�ɫ��ĩA���衢���ˣ��õ��ϴ�����CuSO4��Һ��A�Ļ�ѧʽΪ______________������A��������__________________________��
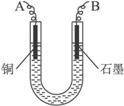
��2�����ϴ�����CuSO4��Һ������ͼ��ʾ��װ���н��е�⣬ʯī�缫�ϵĵ缫��ӦʽΪ_____________����ⷴӦ�����ӷ���ʽΪ__________________________��
��3��ʵ����ɺ�ʯī�缫������״���µ�����V mL��ͭ�缫����a g����Cu�����ԭ���������ô���a��V�ļ���ʽ��ʾ��Ϊ_____________��
��1��CuO ������ҺpH��4��5����ȥFe3+������Һ��ȣ���ȥFe3+Ҳ�ɣ�
��2��4OH-��4e-====O2��+2H2O
2Cu2++2H2O![]() 2Cu+O2��+4H+��3��
2Cu+O2��+4H+��3��![]()
����������������CuO��ĩ��ȥ���Һ�е�H2SO4����ʹFe3+ˮ������Fe(OH)3��������ȥ������Cu�����ԭ������Ӧ���ݵ���������������ת�Ƶ�������ȼ��㡣

��ϰ��ϵ�д�
 �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ