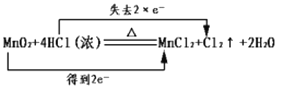
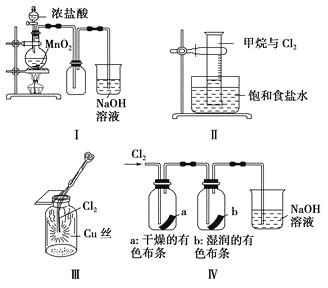
��Ŀ����
����Ŀ��ij�����Ĺ�ҵ��ˮ�к��д�����FeSO4���϶��CuSO4������Na2SO4��Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ���������������ͼ����ɻ�������������ͭ��ʵ�鷽����
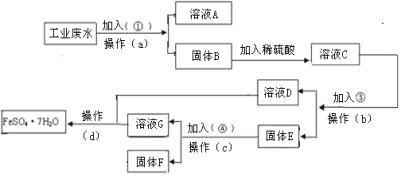
��1����������Լ���ΪNaOH�������Լ��ٵ�Ŀ��___��
��2������a������Ϊ���ˡ�ϴ�ӣ�����Ҫ�IJ�������Ϊ�ձ���___��
��3��������Լ���Ϊ___���ѧʽ�������������ӷ�Ӧ����ʽΪ___��
��4������E�ijɷ�ΪFe��Cu��д��E�ͼ����Լ��������������ӷ�Ӧ����ʽΪ__��
��5������ҺD����ҺG�еõ�FeSO47H2O����IJ���Ϊ����Ũ������ȴ�ᾧ��__��ϴ�ӡ����
���𰸡�ʹCu2+��Fe2+��ȫ���� ©���������� Fe Fe+Cu2+=Fe2++Cu Fe+2H+=Fe2++H2�� ����
��������
�������̣���ˮ�м����Թ�����NaOH��Һ�����˿ɵõ�������ͭ����������������������ϡ�����ܽ⣬�õ���������������ͭ��Һ������ҺC���ټ����������м���ɵõ�����������Һ����ҺD����Cu��Fe�Ļ�����E������E����ϡ���ᣬδ�ܽ��ΪCu����ҺGΪ������������������Ũ������ȴ�ᾧ���ɵõ��̷���
��1���Լ���ΪNaOH����ʹ��Һ�е�Cu2+��Fe2+��ȫ���ɳ�����
��2�����ˡ�ϴ��ʱ����Ҫ�IJ�������Ϊ�ձ���©������������
��3���Լ���ΪFe�����û���Һ�е�Cu2+�������������Ӻ�Cu�����ӷ���ʽΪFe+Cu2+=Fe2++Cu��
��4���Լ���Ϊϡ���ᣬ������Ӧ�����������Ӻ����������ӷ���ʽΪFe+2H+=Fe2++H2����
��5����Һ����ʱ�ķ���Ϊ���ˡ�