��Ŀ����
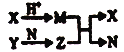
����Ŀ��һ�ְ�ɫ����A��������ˮ����A�����Һ�������¿�ͼ��ʾ��ʵ�飬ʵ������ת����ϵ�����п�ͼ��ʾ��AΪ��������������Ԫ�ػ��ϼ�Ϊ+4������D��ʹƷ����Һ��ɫ������F��ʹʪ��ĺ�ɫʯ����ֽ�������Իش��������⣺

��1��д���������ʻ�ѧʽ��D��__________��F��__________��
��2��д�����з�Ӧ�����ӷ���ʽ��
��A������KMnO4��Һ��Ӧ__________��
��D��������ˮ��__________��
��3��ͨ������Ľ���ڼ���SO42-ʱ��Ӧʹ��__________��
A.�����ữ��BaCl2��Һ B.�����ữ��Ba(NO3)2��Һ
���𰸡� SO2 NH3 2MnO4-+6H++5SO32-=5SO42-+2Mn2++5H2O SO2+Cl2+2H2O=SO42-+4H++2Cl- A
�����������������������֪��������ʹƷ����Һ��ɫ���Ƕ�����������D�Ƕ���������ʹʪ��ĺ�ɫʯ����ֽ�������ǰ�������F�ǰ�����A��һ�ְ�ɫ���壬���������ʷ�Ӧ�������ɰ����������ɶ�����������A��(NH4)2SO3�����ױ�����������Ϊ����泥�����B������泥��ó�E���Ȼ�泥���ɫ����C�����ᱵ��
��1����������������D�Ƕ�������F�ǰ������ʴ�Ϊ��SO2��NH3��
��2����A��(NH4)2SO3��A������KMnO4��Һ��Ӧ�����ӷ���ʽΪ2MnO4-+6H++5SO32-=5SO42-+2Mn2++5H2O��
�������ܽ������������������ӷ���ʽΪ��SO2+Cl2+2H2O=SO42-+4H++2Cl-��
��3��ͨ������Ľ���֪������Һ�д�������������ӣ�����������ܹ�������Ϊ��������ӣ�����ڼ���SO42-ʱ��Ӧʹ�������ữ��BaCl2��Һ����ѡA��

����Ŀ��(l)Ti(BH4)3��һ�ִ�����ϣ�����TiCl4��LiBH4��Ӧ�Ƶá�
�ٻ�̬Ti3+��δ�ɶԵ�������______����
��LiBH4��Li+��BH4-���ɣ�BH4-�Ŀռ乹����________��Bԭ�ӵ��ӻ����������_____________��
��ij��������ǵ������ڽ���Ԫ��M���⻯�M�IJ��ֵ��������±���ʾ:
I1/ kJ��mol-1 | I2/ kJ��mol-1 | I3/ kJ��mol-1 | I4/ kJ��mol-1 | I5/ kJ��mol-1 |
738 | 1451 | 7733 | 10540 | 13630 |
M��_____ (��Ԫ�ط���)��
(2)ͭ������ͭԭ�ӵĶѻ��� ʽ��ͼ1��ʾ��ͭ������ԭ�ӵĶѻ�ģ������______��

(3)Aԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��A�γɻ�����ľ�����ͼ2��ʾ(�ڵ����ͭԭ��)��
�ٸþ���Ļ�ѧʽΪ______________________��
�ڸû�����������ˮ�������ڰ�ˮ����ԭ����______________���˻�����İ�ˮ��Һ��������������Ϊ����ɫ������ɫ��Һ�������ӵĻ�ѧʽΪ_______��
����֪�þ�����ܶ�Ϊpg . cm-3������٤������ΪNA,��֪�þ�����Cuԭ�Ӻ�Aԭ��֮�����̾���Ϊ��Խ��ߵ�1/4����þ�����Cuԭ�Ӻ�Aԭ��֮�����̾���Ϊ__________________pm��
����Ŀ������ͼ��ʾ��һ�ܱ���������Ħ�����ɻ�����������a��b�ֳɼס������ң���״���£��������г���NH30.4mol�������г���HCl��N2�Ļ�����壬��ֹʱ����λ����ͼ��ʾ����֪�ס������������������֮��Ϊ17.3g��
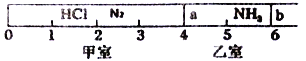
��1��������HCl��N2������֮��Ϊ__________________��
��2��������aȥ����һ��ʱ�����b���ȶ�λ�ڿ̶ȡ�________�������������֣������ǹ������ʲ�����ѹǿ������ʱ��ϵ��ƽ����Է�������Ϊ________��
����֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
���ʵ����ʵ���Ũ��/molL-1 | ��Һ���ܶ�/gcm-3 | |
���� | c1 | ��1 |
��ˮ | c2 | ��2 |
��1�������������������Ϊ________����д��λ���ú�c1����1���Ĵ���ʽ��ʾ����
��2�����ʵ���Ũ��Ϊc1molL-1��������ˮ�������ϣ���Ϻ���Һ������仯���Բ��ƣ���������Һ�����ʵ���Ũ��Ϊ________molL-1��
��3�������ʵ���Ũ�ȷֱ�Ϊc2molL-1��0.2c2molL-1�İ�ˮ��������ϣ�������Һ�����ʵ���Ũ��__________0.6c2molL-1������ڡ�����С�ڡ����ڡ��������Ϻ���Һ������仯���Բ��ƣ�
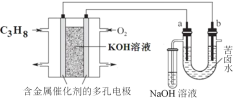
��ⷨ��������������������ɻ������ᣬ���нϸߵĻ���Ч��;���Ч�档ʵ����ģ���ⷨ����NOx��װ����ͼ��ʾ��ͼ�е缫��Ϊʯī�缫����

����NO2�������ģ���ⷨ����ʵ�顣
��д�����ʱNO2������Ӧ�ĵ缫��Ӧʽ��_________________________________��
�����б�״����2.24LNO2�����գ�ͨ�������ӽ���Ĥ��H+Ϊ________mol��
����Ŀ������ʵ���ܵ���Ԥ��Ŀ�ĵ���
ѡ�� | ʵ������ | ʵ��Ŀ�� |
A | ��ע�����ռ�ͭ��Ũ���ᷴӦ���ɵ����壬Ȼ������ע���� | �о�ѹǿ�Ի�ѧƽ���ƶ���Ӱ�� |
B | ��ʵ�����Ƶõ����������м�������̼������Һ��Ȼ������ | ��ȥ���������л��е����ᡢ�Ҵ� |
C | �ñ���FeCl3��Һ�Ƶ�Fe(OH)3���壬Ȼ����� | �ᴿFe (OH) 3���� |
D | ������FeCl2��CuCl2��Һ�м���H2O2 ���ð�ˮ����pH ��2.7 ����� | ��ȥCuCl2��Һ��FeCl2���� |
A. A B. B C. C D. D