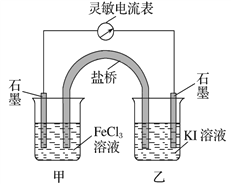
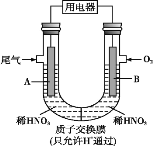
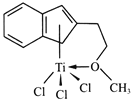
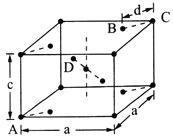
��Ŀ����
����Ŀ��������һ�ּ��߷�չDZ���������Դ����̫����Ϊ���ܣ��Ȼ�ѧ���ѭ���ֽ�ˮ��һ�ָ�Ч������Ⱦ�����ⷽ�����䷴Ӧ��������ͼ��ʾ��

��1����ӦI�Ļ�ѧ����ʽ��________��
��2����ӦI�õ��IJ�����I2���з��롣�ò������Һ�ڹ���I2�Ĵ����»�ֳ����㣬����Ũ��I2��H2SO4��ͺ���Ũ��I2��HI�㡣
�ٸ���������ʵ������˵����ȷ����________��ѡ����ţ���
a ������Һ���ܶȴ��ڲ���
b ��I2ǰ��H2SO4��Һ��HI��Һ������
c I2��HI��Һ�б���H2SO4��Һ������
�� ���������Һ�ķ�����____________��
�۾���⣬H2SO4����c��H+����c��SO42-��=2.06��1�����ֵ����2��ԭ��______��
��3����ӦII �� 2H2SO4��l��=2SO2��g�� +O2��g�� +2H2O��g�� ��H=+550 kJ mol-1
����������Ӧ��ɣ�
i.H2SO4��l��=SO3��g�� +H2O��g�� ��H =+177 kJ mol-1
ii.SO3��g���ֽ⡣
L��L1�� L2����X�ɷֱ����ѹǿ���¶ȣ���ͼ��ʾLһ��ʱ������SO3��g����ƽ��ת������X�ı仯��ϵ��

��д����ӦiiSO3��g���ֽ���Ȼ�ѧ����ʽ��________��
��X��������������_______��
���𰸡�SO2+I2+2H2O=H2SO4+2HI ac �۲���ɫ����ɫ�����HI�㣬��ɫdz����H2SO4�� H2SO4�㺬������HI 2SO3��g��![]() 2SO2��g��+O2��g�� ��H= +196 kJmol-1 ѹǿ
2SO2��g��+O2��g�� ��H= +196 kJmol-1 ѹǿ
��������
��1����ӦIΪ����������ⷢ��������ԭ��Ӧ���������HI����ӦΪSO2+2H2O+I2��H2SO4+2HI��
�ʴ�Ϊ��SO2+2H2O+I2��H2SO4+2HI��
��2����a��������Һ���ܶȴ��ڲ�ų������²㣬��a��ȷ��
b����I2ǰ��H2SO4��Һ��HI��Һ���ܣ���ֲ��أ���b����
c��I2��HI��Һ�б���H2SO4��Һ�����ܣ�����ڲ�ͬ�ܼ����ܽ��Բ�ͬ��������ȡ����ֲ��йأ���c��ȷ��
�ʴ�Ϊ��ac��
�� ���������Һ�ķ����ǣ��۲���ɫ����ɫ���ΪHI�㣬��ɫdz��Ϊ����㣬�ʴ�Ϊ���۲���ɫ����ɫ���ΪHI�㣬��ɫdz��Ϊ����㣻
�۾���⣬H2SO4����c��H+����c��SO42-��=2.06��1�����ֵ����2��ԭ��������к�������HI����HI����������ӣ��ʴ�Ϊ��������к�������HI����HI����������ӣ�
��3�����ݸ�˹���ɣ���ӦII=i��2+ii��ii=II-i��2����ii�ġ�H=+550 kJ��mol-1-��+177 kJ��mol-1����2= +196 kJ��mol-1����ӦiiSO3��g���ֽ���Ȼ�ѧ����ʽ��2SO3��g��![]() 2SO2��g��+O2��g�� ��H= +196 kJ��mol-1 ���ʴ�Ϊ��2SO3��g��
2SO2��g��+O2��g�� ��H= +196 kJ��mol-1 ���ʴ�Ϊ��2SO3��g��![]() 2SO2��g��+O2��g�� ��H= +196 kJ��mol-1��
2SO2��g��+O2��g�� ��H= +196 kJ��mol-1��
��ͼ��֪��XԽ��ת����Խ�ͣ������¶�ת����������X��ʾѹǿ���ʴ�Ϊ��ѹǿ��

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�