��Ŀ����
������Ԫ��X��Y��Z��L��M��Q��ԭ�Ӱ뾶����Ҫ���ϼۼ��±�
| Ԫ�ش��� | X | Y | Z | L | M | Q |
| ԭ�Ӱ뾶/nm | 0��160 | 0��143 | 0��102 | 0��099 | 0��077 | 0��074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | +7��-1 | +4��-4 | -2 |
������6��Ԫ�أ��û�ѧ����ش���������
��1��ͬ�����Ԫ���� ��
��2��д��ͬ����Ԫ�ؼ��γɵij��������ж����廯����ķ���ʽΪ ��
��3��д����ͬ���ڵ�Ԫ�ؼ��γɵij�����ΪҺ����������ˮ������һ�ֻ�����ĵ���ʽ ��
��4����������X��Y�ĵ������������ᷴӦ������H2�����ʵ���֮��Ϊ
��5��д���ɱ���Ԫ���γɵ����ʼ䷢���������û���Ӧ�Ļ�ѧ����ʽ
��1��S O (�����Ⱥ�)��2�֣�
��2�� CO ��2�֣�
��3��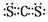 ��
�� 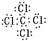 ��2�֣�
��2�֣�
��4��3��4 ��2�֣�
��5��2Mg+ CO2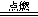 2MgO + C ��2�֣�
2MgO + C ��2�֣�
�����������������Ԫ��ԭ�ӵİ뾶����Ҫ���ϼۿ��жϣ�X��Mg��Y��Al��Z��S��L��Cl��M��C��Q��O��
��1��ͬ�����Ԫ����S��O ��
��2��ͬ����Ԫ�ؼ��γɵij��������ж����廯����ķ���ʽΪCO��
��3����ͬ���ڵ�Ԫ�ؼ��γɵij�����ΪҺ����������ˮ�Ļ�����Ҫô��CCl4��Ҫô��CS2������ʽ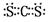 ��
�� 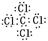 ��
��
��4����������Mg��Al�ĵ������������ᷴӦ��
Mg ~ H2 2Al ~ 3H2
24g 1mol 2��27g 3mol
1g xmol 1g ymol
����H2�����ʵ���֮��Ϊx��y��3��4��
��5������Ԫ���γɵ����ʼ䷢���������û���Ӧ�Ļ�ѧ����ʽ 2Mg+ CO2 2MgO + C��2H2S+O2
2MgO + C��2H2S+O2 2H2O+2S��H2S+Cl2=2HCl+S����
2H2O+2S��H2S+Cl2=2HCl+S����
���㣺����Ԫ�ػ�������ƶϡ�

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�������ѧ��ѧ����Ԫ��ԭ�ӽṹ�����������ʾ��
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�����������г������������������Ȼ����Է����������35.5 |
| �� | B | Bԭ���������������ڲ���������� |
| �� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
| �� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
| �� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
| �� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
��1��Aԭ�������ڱ��е�λ��Ϊ________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ________��������________���������ӡ����ۡ�����
��3��F��E�����γ�10���Ӻ�18���ӵ����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����_______________________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ��__________________________________________________________��
��5���о�һ��ʵ����ʵ֤��A��B����Ԫ�ص��ʻ�ԭ�Ե�ǿ��_______________________________________________________________��

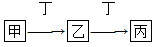
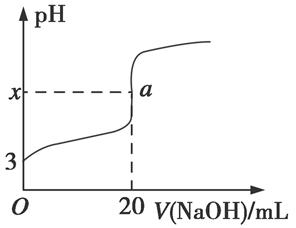
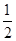 C2��g��=AC2��g������H����283.0 kJ��mol��1
C2��g��=AC2��g������H����283.0 kJ��mol��1