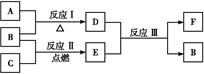
��Ŀ����
ij�̬������W��X��Y��Z 4�ֶ�����Ԫ����ɣ�����W��ԭ�Ӱ뾶��С��
��.��Y��Zͬ���壬ZY2���γ��������Ҫ����֮һ��
��1����X��Y��Z��Ԫ�ط���������ͼ��ʾԪ�����ڱ����ֲ����е���Ӧλ���ϡ�
��2��X������������Ӧˮ�����ϡ��Һ��ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��һ�������£�1 mol XW3������O2��ȫ��Ӧ����XԪ�صĵ��ʺ�Һ̬ˮ���ų�382.8 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ __��
��.��Z���γɻ�������������Ԫ�ء�
��4���õ��ʵ������� __����һ�֣���
��5��HR�Ǻ�ZԪ�ص�һԪ�ᡣ����ʱ����0.250 mol��L��1NaOH��Һ�ζ�25.0 mL HR��Һʱ����Һ��pH�仯�����ͼ��ʾ��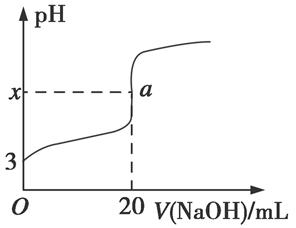
���У�a���ʾ��������ǡ����ȫ��Ӧ��
��ͼ��x �������������������7��
������ʱ��HR�ĵ��볣��Ka�� ������ֵ����
��1����ͼ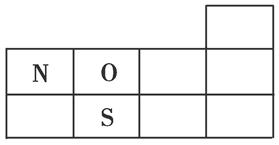
��2��3Cu��8HNO3��ϡ��=3Cu��NO3��2��2NO����4H2O
��3��4NH3��g����3O2��g��=2N2��g����6H2O��l�� ��H����1531.2 kJ��mol��1
��4��̼�����
��5������5.0��10��6
����

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д���֪A��B��C��D��E��F���ֶ�����Ԫ���У�A��B��C��D����ɵ����ʵĻ���Ԫ�أ�A��B��ԭ������֮�͵���Cԭ�Ӻ��ڵ���������A��E��D��F�ֱ�λ��ͬһ���壬��Fԭ�Ӻ��ڵ���������Dԭ�Ӻ����������2�����ݴˣ���ش�
��1��F�����ڱ��е�λ����____________________________��
��2����A��C��D��F��8:2:4:1ԭ�Ӹ�������ɵĻ�������к��еĻ�ѧ������Ϊ____________������Һ�и�����Ũ���ɴ�С��˳��Ϊ________________��������Ũ�ȷ��ű�ʾ����
��3������������A��C�������Է�������Ϊ32�����������A��D����ҷ����ڵ����������ҷ����ڵ���������ȣ�������ķ�Ӧ�����ڻ�����䣨��Ӧ���ﲻ��Ⱦ����������÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
��4����A��D��E��F��ɵĻ����ﶡ�������ᷴӦ���ų��̼�����ζ�����壬�Ļ�ѧʽΪ�ߣߣߣߣߣߣߣߣ�ʵ���ö���Һ�������ԣ��ɴ����ܵó��Ľ�����___________________��
��5����B��A��1:4ԭ�Ӹ�������ɵĻ���������D�ij�����̬���ʼ�NaOH��Һ����ԭ���
|
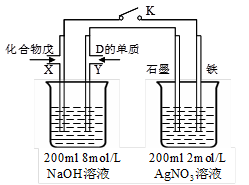
�ٱպ�K��д�����X�缫�ķ�Ӧʽ__________________________________��
�ڱպ�K����X�缫����1.6g��������ʱ��������������κ���ʧ�������ҳ��������ų������ڱ�״���µ����Ϊ_________����
������Ԫ��X��Y��Z��L��M��Q��ԭ�Ӱ뾶����Ҫ���ϼۼ��±�
| Ԫ�ش��� | X | Y | Z | L | M | Q |
| ԭ�Ӱ뾶/nm | 0��160 | 0��143 | 0��102 | 0��099 | 0��077 | 0��074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | +7��-1 | +4��-4 | -2 |
������6��Ԫ�أ��û�ѧ����ش���������
��1��ͬ�����Ԫ���� ��
��2��д��ͬ����Ԫ�ؼ��γɵij��������ж����廯����ķ���ʽΪ ��
��3��д����ͬ���ڵ�Ԫ�ؼ��γɵij�����ΪҺ����������ˮ������һ�ֻ�����ĵ���ʽ ��
��4����������X��Y�ĵ������������ᷴӦ������H2�����ʵ���֮��Ϊ
��5��д���ɱ���Ԫ���γɵ����ʼ䷢���������û���Ӧ�Ļ�ѧ����ʽ
X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �� �� �� Ϣ |
| X | X����������ˮ��������̬�⻯������γ�һ���� |
| Y | ���������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ���� |
| Z | Z��һ�ֺ���������Ϊ27��������Ϊ14 |
| W | ����������Ӧ��ˮ������һ�ֲ�����ˮ����ɫ���� |
��1��Zλ��Ԫ�����ڱ���__________���ڵ�_________�壻Z��ԭ�Ӱ뾶��Y��________�� X�ĵ�һ�����ܱ�Y��________�����С������
��2��W��̬ԭ�ӵĺ�������Ų�ʽΪ____ ____��XH3���ӵķе�ϸߣ��������ԭ��____ ��
��3��X���⻯�X2H4�����Ʊ�����֮һ�ǽ�NaClO��Һ��XH3��Ӧ�Ƶã���д���÷�Ӧ�����ӷ���ʽ ��
��4����֪�������ݣ�4W(s)+ O2(g) = 2W2O(s) ��H =" -337.2" KJ/mol 2W(s)+ O2(g) = 2WO(s) ��H =" -314.6" KJ/mol����W2O��O2��Ӧ����WO���Ȼ�ѧ����ʽ�� ��

 H��a kJ�� mol-1����CO2(g) +C(s)��2CO(g)
H��a kJ�� mol-1����CO2(g) +C(s)��2CO(g)  H2(g) + CO2(g)���ұ�Ϊ�÷�Ӧ�ڲ�ͬ�¶�ʱ��ƽ�ⳣ�����÷�Ӧ��
H2(g) + CO2(g)���ұ�Ϊ�÷�Ӧ�ڲ�ͬ�¶�ʱ��ƽ�ⳣ�����÷�Ӧ��