��Ŀ����
4���±���Ԫ�����ڱ���һ���֣���ش��й����⣺���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��2������������ˮ���������ǿ�����ʵĵ���ʽ
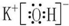 ����ѧ�������Ӽ������ۼ�
����ѧ�������Ӽ������ۼ���3���õ���ʽ��ʾ��Ԫ�����Ԫ���γɻ�����Ĺ���

��4���١��ڡ��ޡ�������Ԫ������������ˮ����������ǿ����HClO4���ѧʽ��
��5����Ԫ�����Ԫ�����ߺ˵����֮����26��
���� ��Ԫ����Ԫ�����ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪF����ΪMg����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr��
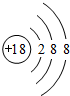
��1��ϡ������Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�
��2������Ԫ����K�Ľ�������ǿ����KOH������ǿ��
��3��Ԫ�آ���Ԫ�آ��γɻ�����ΪMgCl2����þ�����������ӹ��ɣ�
��4���������������ǿ��
��5����Ԫ�����Ԫ�����ߺ˵����֮��Ϊ������������������Ԫ��������
��� �⣺��Ԫ����Ԫ�����ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪF����ΪMg����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr��
��1��ϡ������Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2������Ԫ����K�Ľ�������ǿ����KOH������ǿ�������ʽΪ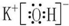 ���������Ӽ������ۼ����ʴ�Ϊ��
���������Ӽ������ۼ����ʴ�Ϊ��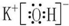 �����Ӽ������ۼ���
�����Ӽ������ۼ���
��3��Ԫ�آ���Ԫ�آ��γɻ�����ΪMgCl2����þ�����������ӹ��ɣ��õ���ʽ��ʾ�γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��4������Ԫ�ص���ۺ�������HClO4��������ǿ���ʴ�Ϊ��HClO4��
��5����Ԫ�����Ԫ�����ߺ˵����֮��Ϊ������������������Ԫ�������������ߺ˵�������8+18=26���ʴ�Ϊ��26��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��Ƚϻ�����ע���õ���ʽ��ʾ��ѧ�������ʵ��γɹ��̣�

| A�� | ������K+��Na+��SO32-��NO3- | B�� | ������K+��Na+��AlO2-��CO32- | ||
| C�� | ��������Na+��NH4+��SiO32-��C1- | D�� | ��ϩ��H+��K+��MnO4-��SO42- |
| A�� | ���������ݻ����¶Ȳ��䣬�����м���1molH2����v��I2������ | |
| B�� | ���������ݻ����¶Ȳ��䣬�����м���1molAr��Ar�����뷴Ӧ������v��I2����С | |
| C�� | ��������ѹǿ���¶Ȳ��䣬�����м���11molAr��Ar�����뷴Ӧ������v��H2����С | |
| D�� | ��������ѹǿ���¶Ȳ��䣬�����м���1molH2��g����1molI2��g������v��H2������ |
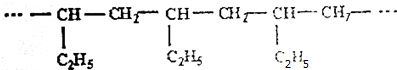
| A�� | CH2=CH-CH3 | B�� | CH2=CH-CH2-CH3 | C�� | CH3-CH=CH-CH3 | D�� | CH2=CH-CH=CH2 |
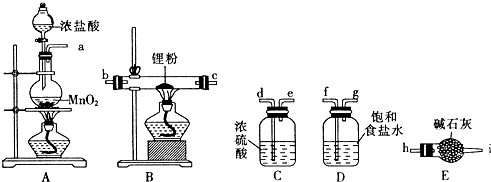

 ��B��G
��B��G
 ��E
��E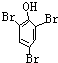 ��
�� ���õ���ʽ��ʾAB2���γɹ���
���õ���ʽ��ʾAB2���γɹ���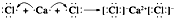 ��
��