��Ŀ����
16��A��B��C��D���ֶ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�B��Dͬ���壮��֪DԪ�������ֳ�����ͬ�������壬CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ���1��AԪ����������CԪ�������ڱ��е�λ�õ������ڵڢ�A�壮
��2��AԪ�صĵ�����ˮ��Ӧ�����ӷ���ʽ��2Na+2H2O=2Na++2OH-+H2����
��3��д��CԪ�صĵ��ʴ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʵĻ�ѧ����ʽCl2+Na2S=2NaCl+S�����÷�Ӧ�ܷ�˵��CԪ�صķǽ����Ա�Bǿ������ܡ�����
��4��BD2��C2��ˮ��Һ������Ư���ԣ����ߵ�Ư��ԭ����ͬ�������ͬ����ͬ����
��5��BԪ�صĵ����ڲ�ͬ�������¿�����O2����һϵ�з�Ӧ��
B��s��+O2��g���TBO2��g����H=-296.8kJ•mol-1
2BO2��s��+O2��g���T2BO3��g����H=-196.6kJ•mol-1
��1mol BO3��g������ȫ�ֽ��B��s������Ӧ�����еķ�Ӧ�ȡ�H=+395.1kJ•mol-1��
���� A��B��C��D���ֶ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�����IA�壬CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ���C�ķǽ����Ա�B��ǿ��B��Dͬ���壬��DԪ�������ֳ�����ͬ�������壬����֪DΪOԪ�ء�BΪSԪ�أ���AΪNa��CΪClԪ�أ��ݴ˽��
��� �⣺A��B��C��D���ֶ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�����IA�壬CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ���C�ķǽ����Ա�B��ǿ��B��Dͬ���壬��DԪ�������ֳ�����ͬ�������壬����֪DΪOԪ�ء�BΪSԪ�أ���AΪNa��CΪClԪ�أ�
��1��������������֪��AΪ�ƣ�CΪ��Ԫ�أ���������Ϊ17�������Ų�����3�����Ӳ㣬����������Ϊ7������λ��Ϊ�������ڵڢ�A�壬
�ʴ�Ϊ���ƣ��������ڵڢ�A�壻
��2��AΪ��Ԫ�أ��ƺ�ˮ��Ӧ�����������ƺ������������ӷ�ӦΪ2Na+2H2O=2Na++2OH-+H2����
�ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����
��3��CԪ�صĵ���Ϊ������A��B��Ԫ����ɵĻ������ˮ��ҺΪNa2S����Һ�������������Դ�����ģ�������ӦΪ��Cl2+Na2S=2NaCl+S��������˵��Cl�ķǽ�����ǿ����
�ʴ�Ϊ��Cl2+Na2S=2NaCl+S�����ܣ�
��4������������Ư��ijЩ��ɫ�������ǻ���������ʱ�ȶ�����ɫ���ʣ�������Ư����������ǿ������ʹ��ɫ��������ɫ����ԭ����ͬ��
�ʴ�Ϊ����ͬ��
��5����֪����S��s��+O2��g���TSO2��g����H=-296.8kJ•mol-1
��2SO2��s��+O2��g���T2SO3��g����H=-196.6kJ•mol-1
���ݸ�˹���ɢ�+�ڡ�$\frac{1}{2}$�ã�S��s��+O2��g���TSO3��g����H=-395.1kJ•mol-1
��1mol SO3��g������ȫ�ֽ��S��s������Ӧ�����еķ�Ӧ�ȡ�H=+395.1kJ•mol-1��
�ʴ�Ϊ��+395.1kJ•mol-1��
���� ���⿼��λ�ýṹ���ʹ�ϵ�ۺ�Ӧ�ã��ƶ�Ԫ���ǽ���ؼ�����5����ע�����ø�˹���ɽ���Ѷ��еȣ�

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�| ��� | c��HCl����mol•L-1�� | �¶ȣ��棩 | ����״̬ |  |
| 1 | 2.0 | 25 | ��״ | |
| 2 | 2.5 | 30 | ��״ | |
| 3 | 2.5 | 50 | ��ĩ״ | |
| 4 | 2.5 | 30 | ��ĩ״ |
| A�� | 3-4-2-1 | B�� | 1-2-4-3 | C�� | 4-3-2-1 | D�� | 1-2-3-4 |
| A�� | �����Dz㣨�ܼ��������������˶��ĵ�����״̬ | |
| B�� | ֻ���ڵ��Ӳ㡢�����Dz㡢�����Ƶ���չ�����ӵ�������ȷ��ʱ�����ӵ��˶�״̬����ȷ������ | |
| C�� | ������B�������ĸ����涼ȷ��ʱ�����ܾ������ÿһ�ܲ��������� | |
| D�� | ��������չ������������С���ص� |
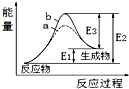
| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | b��ʾ���д��� | |
| C�� | �����ܽ��������淴Ӧ�Ļ�� | |
| D�� | �淴Ӧ�Ļ�ܴ�������Ӧ�Ļ�� |
| A�� | ���ܶ� | B�� | ���۵� | C�� | ���� | D�� | ����� |
| A�� | ���Ȼ�����Һ�м�������İ�ˮ | |
| B�� | ��ϡ�����е���������NaAlO2��Һ | |
| C�� | ��ϡ�����м������� | |
| D�� | �������ữ��MgSO4��Һ�м��������Ba��OH��2��Һ |
| A�� | �����ʵ���Ũ�ȵ�Na2 C O3��NaHCO3���Һ�У�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�� | |
| B�� | 25��ʱ 0.2 mol•L-1����������0.05 mol•L-1Ba��OH��2��Һ��Ϻ���Һ��pH=l | |
| C�� | pH=3�Ķ�Ԫ����H2R��Һ��pH=ll��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ�У�2c��R2-��ʮc��HR-��=c��Na+�� | |
| D�� | 25��ʱ����0.3 mol•L-1 HY��Һ��0.3 mol��L-lNaOH��Һ�������Ϻ���Һ�� pH=9����c��OH-��-c��HY��-c��H+��=1��lO-9 mol•L-1 |
| A�� | Cl2��HCl��������ʳ��ˮ | B�� | H2��H2S��HCl��H2O������ʯ�� | ||
| C�� | SO2��HCl����Na2SO3��Һ | D�� | CO2��H2S����CuSO4��Һ |
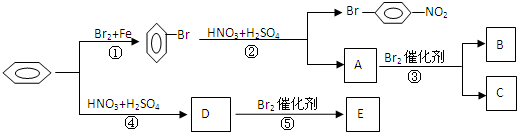
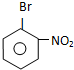 B
B ��
�� C
C E
E
 ��
��