��Ŀ����
5�������е������Һ������������ǣ�������| A�� | �����ʵ���Ũ�ȵ�Na2 C O3��NaHCO3���Һ�У�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�� | |
| B�� | 25��ʱ 0.2 mol•L-1����������0.05 mol•L-1Ba��OH��2��Һ��Ϻ���Һ��pH=l | |
| C�� | pH=3�Ķ�Ԫ����H2R��Һ��pH=ll��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ�У�2c��R2-��ʮc��HR-��=c��Na+�� | |
| D�� | 25��ʱ����0.3 mol•L-1 HY��Һ��0.3 mol��L-lNaOH��Һ�������Ϻ���Һ�� pH=9����c��OH-��-c��HY��-c��H+��=1��lO-9 mol•L-1 |
���� A��̼������ӵ�ˮ��̶ȴ���̼��������ӣ�����Һ��̼���������Ũ�ȴ���̼������ӣ���Һ��ʾ���ԣ�
B�����Һ�������ӵ�Ũ��Ϊ��$\frac{0.2mol/L-0.05mol/L��2}{2}$=0.05mol/L��
C����Һ��pH=7����c��OH-��=c��H+�������ݻ��Һ�е���غ��жϣ�
D�����ݻ��һ�ֵĵ���غ�������غ���㣮
��� �⣺A�������ʵ���Ũ�ȵ�Na2CO3��NaHCO3���Һ�У�CO32-��ˮ��̶ȴ���HCO3-����c��HCO3-����c��CO32-����c��OH-����c��H+������Һ������Ũ�ȴ�СΪ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+������A��ȷ��
B.25��ʱ 0.2 mol•L-1����������0.05 mol��L-1Ba��OH��2��Һ��Ϻ������ӵ�Ũ��Ϊ��$\frac{0.2mol/L-0.05mol/L��2}{2}$=0.05mol/L����Һ��pH��1����B����
C��pH=3�Ķ�Ԫ����H2R��Һ��pH=ll��NaOH��Һ��Ϻ��Һ��pH����7����c��OH-��=c��H+�������ݵ���غ�ɵã�2c��R2-��ʮc��HR-��=c��Na+������C��ȷ��
D�����������غ㣬Ӧ��c��HY��+c��Y-��=c��Na+�������ݵ���غ�Ӧ��c��Na+��+c��H+��=c��Y-��+c��OH-����������ʽ�ɵã�c��HY��+c��H+��=c��OH-������c��OH-��-c��HY��=c��H+��=1��10-9mol•L-1����D����
��ѡBD��
���� ���⿼������ϵĶ����ж��Լ�����Ũ�ȴ�С�Ƚ����⣬��Ŀ�Ѷ��еȣ�ע�����������Ϣ�ж�����ˮ����ص㣬����غ�˼������⣮

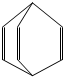 ϩ��������װ����ʱ���õ����ʣ�����Ͱϩ�������б���������һ�֣���ṹ��ʽ��ͼ��ʾ���й�Ͱϩ˵������ȷ���ǣ�������
ϩ��������װ����ʱ���õ����ʣ�����Ͱϩ�������б���������һ�֣���ṹ��ʽ��ͼ��ʾ���й�Ͱϩ˵������ȷ���ǣ�������| A�� | ������Ͱϩ���������ש�ϣ����û� | |
| B�� | 1molͰϩ��һ������������3molCl2�����ӳɷ�Ӧ | |
| C�� | Ͱϩ�뱽��ϩ��Ϊͬ���칹�� | |
| D�� | Ͱϩ�����������л�Ϳ�ϵ��ܼ� |
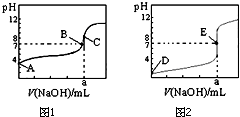 ��ͼ1��2Ϊ��������0.10mol•L-1 NaOH��Һ�ζ�20.00mL 0.10mol•L-1 �����20.00mL 0.10mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������
��ͼ1��2Ϊ��������0.10mol•L-1 NaOH��Һ�ζ�20.00mL 0.10mol•L-1 �����20.00mL 0.10mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������| A�� | ͼ1�ǵζ���������� | |
| B�� | E��ʱ��Һ������Ũ��Ϊc��Na+ ��=c��A- �� | |
| C�� | B��ʱ����Ӧ������Һ�����V��NaOH����V��HA�� | |
| D�� | ��0mL��V��NaOH����20.00mLʱ����Һ��һ����c��A-����c��Na+ ����c��H+ ����c��OH- �� |
| A�� | �ñ����Ȼ����Һ������ϴ����������� | |
| B�� | NOx��Cl2��PM2.5�������ᵼ������ | |
| C�� | ��ʳƷ���з���ʢ�й轺����С�����ɷ�ֹʳ���ܳ� | |
| D�� | ������������������������Գմ������������˴�������ˮ�� |
| A�� | pH=5��H2S��Һ�У�c��H+��=c��HSһ��=1��10-5mol•L-1 | |
| B�� | ����AgCl��AgI���������Һ�У�c��Ag+����c��Cl-��=c��I-�� | |
| C�� | ��������ˮ�м����Ȼ��ƹ��壬ˮ�ĵ���ƽ�ⲻ�ƶ� | |
| D�� |  ��RΪZn������ͼ�������������������������� |
| A�� | ��NH4Cl������Һ�м������þ���Եõ��������� | |
| B�� | pH=12Ba��OH��2��Һ��c��OH-����0.001mol/LNaOH��Һc��OH-����10�� | |
| C�� | �����£�CH3COONa��CH3COOH�Ļ����Һ�У�pH=7����c��Na+��=c��CH3COO-����c��CH3COOH����c��H+��=c��OH-�� | |
| D�� | �����£�Cd��OH��2��Co��OH��2�Ļ������Һ�У�c��Cd2+����c��Co2+����4������֪��KSP[Cd��OH��2]=7.2��10-15��Ksp[Co��OH��2]=1.8��10-15 |
 �������ƣ�NaNO2����ۿ���ʳ��������ζ����һ�ֳ��õķ�ɫ���ͷ���������ʹ�ù�����ʹ���ж���ijѧϰС����������������������ʵ�飺
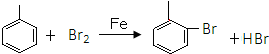
�������ƣ�NaNO2����ۿ���ʳ��������ζ����һ�ֳ��õķ�ɫ���ͷ���������ʹ�ù�����ʹ���ж���ijѧϰС����������������������ʵ�飺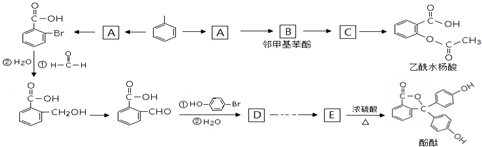
 ��
��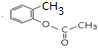 ��E�����еĺ�������������Ϊ�Ȼ����ǻ���
��E�����еĺ�������������Ϊ�Ȼ����ǻ���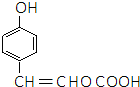 ��
��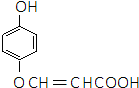 ���ڡ��䡢�Ե�����һ�֣�
���ڡ��䡢�Ե�����һ�֣�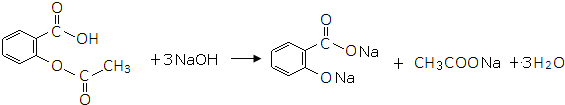 ��
��