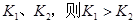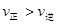��Ŀ����
�ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ�������з�Ӧ��
2N2 (g)+6H2O(l) 4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
������ӦNH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
��1���˺ϳɷ�Ӧ��a 0 (�����������������)��
��2����ˮϡ��0.1 mol��L��1��ˮ����ϡ��ʱ��Һ�¶Ȳ��䣩������Һ������ˮ�������Ӷ���С�������е� ������ţ���
A��c(NH3��H2O) B��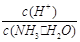 C��c(H+)��c(OH��) D��
C��c(H+)��c(OH��) D��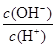
��3����ҵ�ð���ȡ�����������ӦΪ��4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
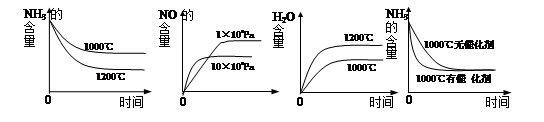
A B C D
��4����1L�ݻ��̶����ܱ������з���������Ӧ���������ʵ����ʵ���Ũ�����±���
��Ӧ�ڵ�6 min��8minʱ�ı����������ı������������___________________���ڸ������£�ƽ����_______�ƶ�(����ҡ�)��
2N2 (g)+6H2O(l)
 4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1������ӦNH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| �¶� T/K | 303 | 313 | 323 |
| NH3������/(10��6 mol) | 4.8 | 5.9 | 6.0 |
��2����ˮϡ��0.1 mol��L��1��ˮ����ϡ��ʱ��Һ�¶Ȳ��䣩������Һ������ˮ�������Ӷ���С�������е� ������ţ���
A��c(NH3��H2O) B��
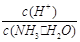 C��c(H+)��c(OH��) D��
C��c(H+)��c(OH��) D��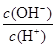
��3����ҵ�ð���ȡ�����������ӦΪ��4NH3(g)+5O2(g)
 4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��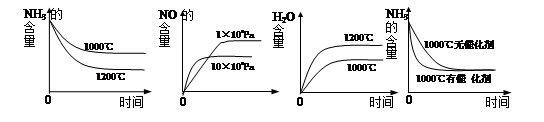
A B C D
��4����1L�ݻ��̶����ܱ������з���������Ӧ���������ʵ����ʵ���Ũ�����±���
| ʱ��/Ũ�� | c(NH3) (mol/L) | c(O2 ) (mol/L) | c(NO) (mol/L) |
| ��ʼ | 0.8000 | 1.600 | 0.000 |
| ��4 min | 0.3000 | 0.9750 | 0.5000 |
| ��6 min | 0.3000 | 0.9750 | 0.5000 |
| ��8 min | 0.7000 | 1.475 | 0.1000 |
��Ӧ�ڵ�6 min��8minʱ�ı����������ı������������___________________���ڸ������£�ƽ����_______�ƶ�(����ҡ�)��
��1����1�֣� �� ��2����2�֣� AD ��3����2�֣�BD
��4����3�֣������¶ȣ�������ѹǿ��2��) �� (1��)
��4����3�֣������¶ȣ�������ѹǿ��2��) �� (1��)
�����������1���ӱ��п��Կ����������¶ȵ����ߣ�ƽ�ⳣ��Խ��Խ��˵����Ӧ������У�����ƽ���ƶ�ԭ������֪���������ȵķ���Ҳ����˵a��0 ����2����ˮϡ�ͣ����ƽ�������漰�������е����ʵ�Ũ�ȶ���С������ƽ�������ƶ�����Ũ�ȼ�С����Ҫ�ģ��ƶ�ֻ�Ǽ������ָı䣬���������������ѡAD�� ��3��A�������¶ȣ�ƽ�������ƶ������ĺ������ӣ�����B����ȷ��C�������¶ȣ�ƽ�������ƶ���ˮ�ĺ�����С������D����ȷ��

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
 SO3(g)+NO(g) ��H��0
SO3(g)+NO(g) ��H��0 2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����
2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����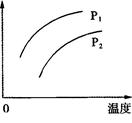
 ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
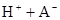

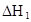
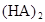
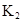
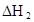
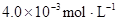
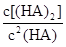 ��130����Ӧ��___________������С�
��130����Ӧ��___________������С� xC(g) ��H �� 0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ����
xC(g) ��H �� 0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ����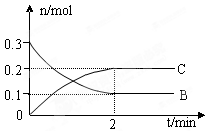
 2HI (g)�ﵽƽ��ı�־��
2HI (g)�ﵽƽ��ı�־��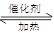 2SO3 ��H��0������������ʵ�������0��5molʱ��Ӧ�ﵽƽ�⡣����������ȷ����
2SO3 ��H��0������������ʵ�������0��5molʱ��Ӧ�ﵽƽ�⡣����������ȷ���� cC + Q��ij�¶��µ�ƽ�ⳣ��ΪK��K��1������Ӧ��ΪQ�������¶Ȳ��䣬������ʽ����д�����¸ı䣬��Q��K��ֵ����Ӧ�仯Ϊ( )
cC + Q��ij�¶��µ�ƽ�ⳣ��ΪK��K��1������Ӧ��ΪQ�������¶Ȳ��䣬������ʽ����д�����¸ı䣬��Q��K��ֵ����Ӧ�仯Ϊ( )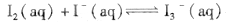 ��ijI2����KI�����Һ�У� c(
��ijI2����KI�����Һ�У� c(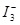 )���¶�T��ƽ������ͼ���¡�����˵������ȷ����
)���¶�T��ƽ������ͼ���¡�����˵������ȷ����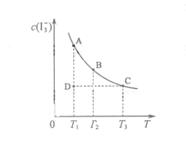
 ����Ӧ��ƽ�ⳣ���ֱ�Ϊ
����Ӧ��ƽ�ⳣ���ֱ�Ϊ