��Ŀ����
ij�о�С���һԪ�л�����HA���ܼ�����ˮ�Ļ����ϵ�е��ܽ�̶Ƚ����о�����25��ʱ������HA��ˮ�в��ֵ��룬��HAŨ��Ϊ ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
�ش��������⣺
��1������25��ʱˮ��Һ��HA�ĵ���ƽ�ⳣ��K1��___________��
��2��25�棬��ˮ��Һ��pHΪ___________������֪��1g2��0.3��lg3��0.5���ڱ���ϵ��HA��ת����Ϊ___________��
��3���ڱ��У�HA�������ۣ�2HA ��HA��2����Ӧ�ڽϵ��¶����Է����У���
��HA��2����Ӧ�ڽϵ��¶����Է����У���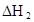 ___________0��
___________0��
��4��25������ϵ�У�HA�ڱ��з������ۣ������ijʱ����Һ����Ũ������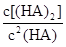 ��130����Ӧ��___________������С�
��130����Ӧ��___________������С�
 ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�| 25��ƽ����ϵ | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
��ˮ�У�HA 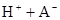 |  | 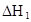 |  |
�ڱ��У�2HA 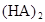 | 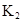 | 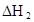 | 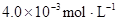 |
�ش��������⣺
��1������25��ʱˮ��Һ��HA�ĵ���ƽ�ⳣ��K1��___________��
��2��25�棬��ˮ��Һ��pHΪ___________������֪��1g2��0.3��lg3��0.5���ڱ���ϵ��HA��ת����Ϊ___________��
��3���ڱ��У�HA�������ۣ�2HA
 ��HA��2����Ӧ�ڽϵ��¶����Է����У���
��HA��2����Ӧ�ڽϵ��¶����Է����У���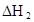 ___________0��
___________0����4��25������ϵ�У�HA�ڱ��з������ۣ������ijʱ����Һ����Ũ������
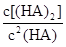 ��130����Ӧ��___________������С�
��130����Ӧ��___________������С���1��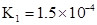 mol/L ��2��3.2�� 40% ��3���� ��4����
mol/L ��2��3.2�� 40% ��3���� ��4����
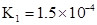 mol/L ��2��3.2�� 40% ��3���� ��4����
mol/L ��2��3.2�� 40% ��3���� ��4���������������1��HA��ˮ��Һ�е��룺HA
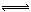 H++A-�����ݵ����Ϊ0.20��c��H+��=3.0��10-3mol/L��0.20=6.0��10-4mol?L?1����K=6.0��10-4mol?L?1��6.0��10-4mol?L?1�£�3.0��10-3mol?L?1��6.0��10-4mol?L?1��=1.5��10-4mol?L?1����2��pH=-lg��H+��=-lg��6.0��10-4��=3.2���ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��c��HA��=3.0��10-3mol?L?1��6.0��10-4mol?L?1=2.4��10-3mol?L?1��ת����HAΪ4.0��10-3mol?L?1��2.4��10-3mol?L?1=1.6��10-3mol?L?1�������ڱ���ϵ��HA��ת����Ϊ,1.6��10-3mol?L?��4.0��10-3mol?L?1��100% ="40%" ����3���÷�Ӧ�ڽϵ��¶����Է����У�T��Сʱ��?H-T?S<0����Ϊ��Ӧ���ʵ�����С����?S<0������?H<0����4���ɣ�2�������ݿ����2HA
H++A-�����ݵ����Ϊ0.20��c��H+��=3.0��10-3mol/L��0.20=6.0��10-4mol?L?1����K=6.0��10-4mol?L?1��6.0��10-4mol?L?1�£�3.0��10-3mol?L?1��6.0��10-4mol?L?1��=1.5��10-4mol?L?1����2��pH=-lg��H+��=-lg��6.0��10-4��=3.2���ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��c��HA��=3.0��10-3mol?L?1��6.0��10-4mol?L?1=2.4��10-3mol?L?1��ת����HAΪ4.0��10-3mol?L?1��2.4��10-3mol?L?1=1.6��10-3mol?L?1�������ڱ���ϵ��HA��ת����Ϊ,1.6��10-3mol?L?��4.0��10-3mol?L?1��100% ="40%" ����3���÷�Ӧ�ڽϵ��¶����Է����У�T��Сʱ��?H-T?S<0����Ϊ��Ӧ���ʵ�����С����?S<0������?H<0����4���ɣ�2�������ݿ����2HA ��HA��2��ƽ�ⳣ��K=8.0��10-4mol?L?1�£�2.4��10-3mol?L?1��2=146��
��HA��2��ƽ�ⳣ��K=8.0��10-4mol?L?1�£�2.4��10-3mol?L?1��2=146��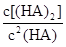 ��130<K�����Է�Ӧ������Ӧ������С�
��130<K�����Է�Ӧ������Ӧ������С�
��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
 2NO
2NO 4NO��g����6H2O��g�� ��H��0�����д�ʩ��ʹƽ�������ƶ�����
4NO��g����6H2O��g�� ��H��0�����д�ʩ��ʹƽ�������ƶ�����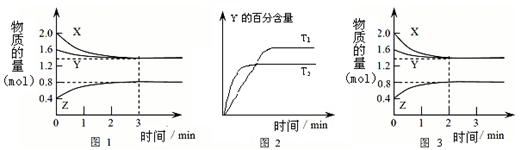
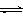 2Z(g)
2Z(g)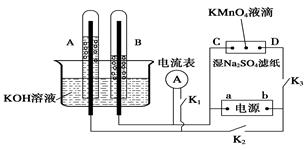
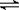 CH3OH(g) ��H���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)����ش��������⣺
CH3OH(g) ��H���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)����ش��������⣺ 4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1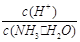 C��c(H+)��c(OH��) D��
C��c(H+)��c(OH��) D��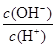
 3 C(g)�ﵽƽ��ʱ�����c(A)��0.5 mol/L�����¶Ȳ��������£��������������һ�������ﵽ�µ�ƽ��ʱ�����c(A)��0.20mol/L�������ж�����ȷ����
3 C(g)�ﵽƽ��ʱ�����c(A)��0.5 mol/L�����¶Ȳ��������£��������������һ�������ﵽ�µ�ƽ��ʱ�����c(A)��0.20mol/L�������ж�����ȷ���� 2C(g) ��H=��QkJ/mol���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1 mol A��3 mol B����һ�������´ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�������£����������м���2 mol C�ﵽƽ�����������ΪQ2 kJ����֪Q1=3Q2��������������ȷ���ǣ� ��
2C(g) ��H=��QkJ/mol���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1 mol A��3 mol B����һ�������´ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�������£����������м���2 mol C�ﵽƽ�����������ΪQ2 kJ����֪Q1=3Q2��������������ȷ���ǣ� ��