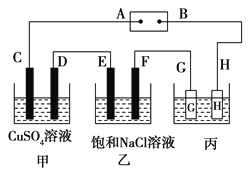
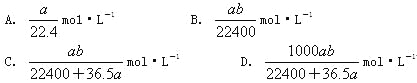��Ŀ����
����Ŀ����t ��ʱ����a g NH3��ȫ����ˮ���õ�V mL��Һ���������Һ���ܶ�Ϊ�� g��mL��1�����ʵ���������Ϊw�����к���NH4+�����ʵ�����b mol������������ȷ���ǣ� ��
A. ���ʵ���������w��![]() ��100%
��100%
B. ���ʵ����ʵ���Ũ��c��![]() mol��L��1
mol��L��1
C. ��Һ��c(OH��)��![]() mol��L��1��c(H��)
mol��L��1��c(H��)
D. ��������Һ�м���V mLˮ��������Һ��������������0.5w
���𰸡�C
��������A����ˮ������Ϊ����������Һ���ܶ�Ϊ�� g��mL��1�����ΪV mL��������Һ����Ϊ��V g�����ʰ���������Ϊa g�����ʵ���������![]() ��100%��
��100%��![]() ��100%��ѡ��A����ȷ��B��a g NH3�����ʵ���Ϊ
��100%��ѡ��A����ȷ��B��a g NH3�����ʵ���Ϊ![]() mol����Һ���ΪV mL���������ʵ����ʵ���Ũ��Ϊ
mol����Һ���ΪV mL���������ʵ����ʵ���Ũ��Ϊ![]() ��
��![]() mol��L��1��ѡ��B����ȷ��C��V mL��Һ��c(OH��)��c(H��)��c(NH
mol��L��1��ѡ��B����ȷ��C��V mL��Һ��c(OH��)��c(H��)��c(NH![]() )��c(H��)��
)��c(H��)��![]() mol��L��1��ѡ��C��ȷ��D�����ǰ�����ʵ��������䣬��Ϊa g����ˮ���ܶȱȰ�ˮ���ܶȴ������ˮ�������Ȱ�ˮ�Ĵ�Ϻ���Һ����������2��V g�����Ի�Ϻ����ʵ���������С��0.5w��ѡ��D����ȷ����ѡC��
mol��L��1��ѡ��C��ȷ��D�����ǰ�����ʵ��������䣬��Ϊa g����ˮ���ܶȱȰ�ˮ���ܶȴ������ˮ�������Ȱ�ˮ�Ĵ�Ϻ���Һ����������2��V g�����Ի�Ϻ����ʵ���������С��0.5w��ѡ��D����ȷ����ѡC��

����Ŀ���������ҹ��������������������2.5�����µ�ϸ������(PM2.5)�ǵ�������������������������������е�CO��SO2�������������Ⱦ�����ͨ��������ѧ��Ӧ����PM2.5�����
��1�� ��CaSO4����O2��ȼ��CO��Ӧ����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ������ȿ����ȼ��Ч�ʣ����ܵõ��ϴ���CO2���Ա��ڱ���������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
����CaSO4(s)��4CO(g)==CaS(s)��4CO2(g) ��H1����189.2 kJ��mol-1
����CaSO4(s)��CO(g)==CaO(s)��CO2(g)��SO2(g)�� ��H2����210.5 kJ��mol-1
����CO(g)==![]() C(s)��
C(s)��![]() CO2(g) ��H3����86.2 kJ��mol-1
CO2(g) ��H3����86.2 kJ��mol-1
��Ӧ2CaSO4(s)��7CO(g)==CaS(s)��CaO(s)��6CO2(g)��C(s)��SO2(g)����H��_________________
��2����֪��CO����CO2�Ļ�ѧ����ʽΪCO��O2![]() CO2��O��������Ӧ����Ϊv��=K����c(CO) ��c(O2)���淴Ӧ����Ϊv��=K����c(CO2) ��c(O)��K����K��Ϊ���ʳ�������2500 K����K��=1.21��105 L��s-1��mol-1��K��=3.02��105 L��s-1��mol-1������¶���������Ӧ��ƽ�ⳣ��KֵΪ________(����С�����һλС��)��
CO2��O��������Ӧ����Ϊv��=K����c(CO) ��c(O2)���淴Ӧ����Ϊv��=K����c(CO2) ��c(O)��K����K��Ϊ���ʳ�������2500 K����K��=1.21��105 L��s-1��mol-1��K��=3.02��105 L��s-1��mol-1������¶���������Ӧ��ƽ�ⳣ��KֵΪ________(����С�����һλС��)��
��3���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������Ӧ��C(s)��2NO(g)![]() N2(g)��CO2(g)����H=Q kJ��mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2(g)����H=Q kJ��mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ��(min) Ũ��(mol��L-1) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
��0��10 min�ڣ�NO��ƽ����Ӧ����v(NO)=___________________________________��
��30 min��ֻ�ı�ijһ��������Ӧ����ƽ�⣬�����ϱ������жϸı������������____(ѡ����ĸ)��
a������һ�����Ļ���̿ b��ͨ��һ������NO c���ʵ���С��������� d��������ʵĴ���
����30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����Q_____0 (������������������������)��
��4��������ͼ��ʾ���װ��(�缫��Ϊ���Ե缫)Ҳ������SO2�������������ų�����Һ����NO2�����Դb�����ӵĵ缫�ĵ缫��ӦʽΪ____________________________________��
��5��NO2��һ�������¿�ת��ΪNH4NO3��NH4NO2����ͬ�¶�������Ũ��NH4NO3��NH4NO2������Һ�����NH4NO2��Һ��c(NH4+)��С���������ܵ�ԭ��________________________��
����Ŀ��700��ʱ��H2��g����CO2��g��![]() H2O��g����CO��g�������¶��£��ڼס��ҡ������������ܱ������У�Ͷ��H2��CO2����ʼŨ�����±���ʾ�����м�2min��ƽ��ʱ��v (H2O)Ϊ0.025 mol/��L��min���������жϲ���ȷ���ǣ� ��
H2O��g����CO��g�������¶��£��ڼס��ҡ������������ܱ������У�Ͷ��H2��CO2����ʼŨ�����±���ʾ�����м�2min��ƽ��ʱ��v (H2O)Ϊ0.025 mol/��L��min���������жϲ���ȷ���ǣ� ��
��ʼŨ�� | �� | �� | �� |
C��H2��/mol/L | 0.1 | 0.2 | 0.2 |
C��CO2��/mol/L | 0.1 | 0.1 | 0.2 |
A. ƽ��ʱ������CO2��ת���ʴ���50��
B. ����Ӧƽ��ʱ������c��CO2���Ǽ��е�2��
C. �¶�����800�棬������Ӧƽ�ⳣ��Ϊ25/16��������ӦΪ���ȷ�Ӧ
D. �����������䣬����ʼʱ���������г���0.10mol/L H2��0.20 mol/L CO2������ƽ��ʱc (CO)���Ҳ�ͬ