��Ŀ����
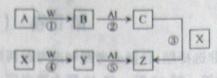
��16�֣����������Ҫ�ɷ�ΪAl2O3�����������[NH4Al(SO4)2��12H2O]��Z����ˮ��Һ�������˿��������Ĺ�������ͼ���£�
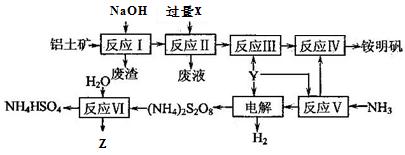
��1����Ӧ�����Ҫ���ӷ���ʽ��__________________________ ��
��2����֪��Ӧ���л�����������������������������XΪ_________���ѧʽ����
��3������⡱ʱ���ö��Բ������缫����������Ӧ�ɱ�ʾΪ _________ ��
��4�������п���ѭ��ʹ�õ������� ��
��5����Ӧ���Ļ�ѧ����ʽΪ __________ �����еĻ�ԭ����____________��
��6����֪�������������;���������ƣ���˵��������ľ�ˮԭ��_________________________ ��

��1����Ӧ�����Ҫ���ӷ���ʽ��__________________________ ��
��2����֪��Ӧ���л�����������������������������XΪ_________���ѧʽ����
��3������⡱ʱ���ö��Բ������缫����������Ӧ�ɱ�ʾΪ _________ ��
��4�������п���ѭ��ʹ�õ������� ��
��5����Ӧ���Ļ�ѧ����ʽΪ __________ �����еĻ�ԭ����____________��
��6����֪�������������;���������ƣ���˵��������ľ�ˮԭ��_________________________ ��
��1��Al2O3+2OH��=2AlO2��+H2O��2�֣�
��2��CO2 �����������𰸣�2�֣�
��3��2H����2e�� = H2����2�֣�
��4��NH4HSO4��2�֣�
��5��(NH4)2S2O8��2H2O = 2NH4HSO4��H2O2 ��3�֣� H2O ��2�֣�
��6���������ˮ�л�����Al3+��Al3+ˮ������Al(OH)3���壬Al3++3H2O Al(OH)3+3H+��Al(OH)3�����ܹ�����ˮ�е���������ˮ���á���3�֣�
Al(OH)3+3H+��Al(OH)3�����ܹ�����ˮ�е���������ˮ���á���3�֣�
��2��CO2 �����������𰸣�2�֣�
��3��2H����2e�� = H2����2�֣�
��4��NH4HSO4��2�֣�
��5��(NH4)2S2O8��2H2O = 2NH4HSO4��H2O2 ��3�֣� H2O ��2�֣�
��6���������ˮ�л�����Al3+��Al3+ˮ������Al(OH)3���壬Al3++3H2O
 Al(OH)3+3H+��Al(OH)3�����ܹ�����ˮ�е���������ˮ���á���3�֣�
Al(OH)3+3H+��Al(OH)3�����ܹ�����ˮ�е���������ˮ���á���3�֣���

��ϰ��ϵ�д�
�����Ŀ
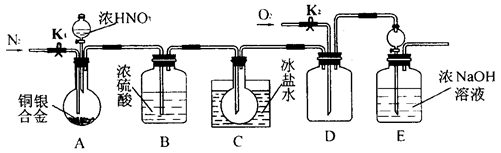

 �Թ�B�е�������___________________��
�Թ�B�е�������___________________��
 �������ӷ���ʽ��ʾ����
�������ӷ���ʽ��ʾ����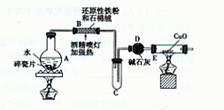
 FeSO4��Һ
FeSO4��Һ FeSO4��7H2O����
FeSO4��7H2O����
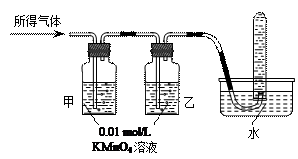
 ������������ȷ��̼��Ƶ�����������
������������ȷ��̼��Ƶ�����������
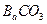 ������ֻҪ�ⶨװ��C������
������ֻҪ�ⶨװ��C������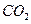 ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.
ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.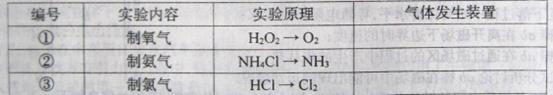
 ��
�� װ���Ʊ�O
װ���Ʊ�O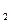 ����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O
����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O ���У�д����Ӧ�ܵ����ӷ���ʽ�� ��
���У�д����Ӧ�ܵ����ӷ���ʽ�� �� ������100mL��
������100mL��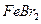 ��Һ��ͨ���״����Cl
��Һ��ͨ���״����Cl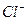 ��
��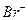 �����ʵ���Ũ����ȣ���ͨ��Cl
�����ʵ���Ũ����ȣ���ͨ��Cl